Grade 11 Chem Unit Test #1
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
Physical Properties—Qualitative
-State
-Colour
-Clarity—transparent, translucent, opaque
-Viscosity
-Odour
-Taste
-Crystal, powder, granular
-Shiny/dull (lustrous)
-Ductile
-Malleable
-Texture
-Mixture
Physical Properties—Quanitative
-Volume
-Mass
-Density
-Temperature
-pH
-Viscosity
-Conductivity
Chemical Properties
-react with acid
-react with base
-combustibility (react with O2)
-rust/ oxide (react with O2)
Physical Changes
-change of state (phase change)
-dissolving
-form change
E.x., evaporation, salt + water, bending lead
Chemical Changes
-heat/ light released/ absorbed
-colour change
-precipitate (new solid)
-gas (bubbles, vapors, new smell)
-can’t reverse
Groups
Vertical columns
Periods
Horizontal rows
What are diatomic molecules? Identify seven of these.
Ions with more than one atom
H2 O2 Br2 F2 I2 N2 Cl2
Acids
pH value: 1-6 or <7
Colour in phenolphthalein: colourless
Colour of litmus paper: red
Ions present in solution: H+
Physical properties: grippy, sour, corrosive, conductive, aqueous
Bases
pH value: 8-14 or >7
Colour in phenolphthalein: pink
Colour of litmus paper: blue
Ions present in solution: OH-
Physical properties: bitter, corrosive, conductive, aqueous, slippery
Atom
The smallest particle of an element
Atomic Model & Theory Timeline
Democritus: he proposed that matter could not be divided into smaller pieces forever. He claimed that matter was made of small, hard particles that he called “atoms”.
Dalton: he created the very first atomic theory. Dalton viewed atoms as tiny, solid balls.
Thomson: he showed that the atom was made of even smaller things. His atomic model was known as the “raisin bun model”
Rutherford: he discovered protons and the nucleus. He showed that atoms have (+) particles in the center, and are mostly empty space.
Bohr: he improved on Rutherford’s model. He proposed that electrons move around the nucleus in specific layers, or shells.
Chadwick: he discovered neutrons. Working with Rutherford, he discovered particles with no charge; these particles were called as neutrons.
Modern: work done since 1920 has changed the model. The new atomic model has electrons moving around the neon a cloud.
Democritus
The smallest bit of matter was an atom
Dalton
Described all matter as being composed of tiny particles called atoms, represented by different sized spheres
Thomson
“Plum pudding” in which a positively charged sphere had negatively charged particles embedded in it
Rutherford
Planetary model in which all the positive charge and most of the mass of the atom is located in the center of atom, with the electrons orbiting the nucleus
Bohr
Modified planetary model, stating electrons have specific amounts of energy and occupy orbits, or energy level
Quantum mechanical model
Determined the electrons not found in precise orbit, but existed in electron clouds called orbitals
Electron
Location in the atom: in orbit
Mass: 1/2000 u
Charge: -
Proton
Location in atom: in nucleus
Mass: 1 u
Charge: +
Neutron
Location in atom: in nucleus
Mass: 1 u
Charge: o
Chemical Notation
A—mass # (p+, n0)
X—chemical symbol
Z—atomic # (p+, e-)
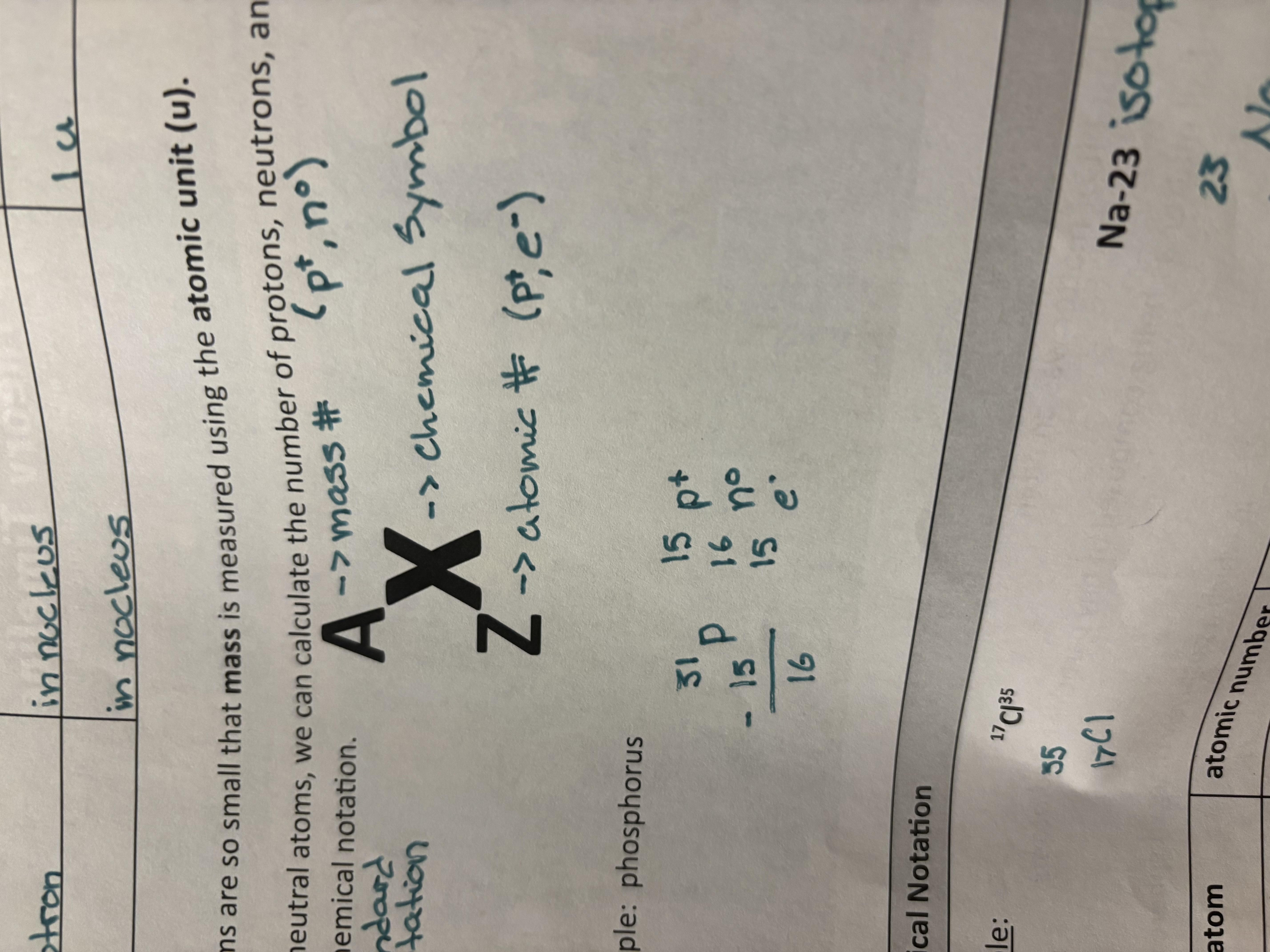
Isotope
A form of a chemical element where atoms have the same number of protons but a different number of neutrons
Ion
When an atom loses or gains electrons and becomes charged
Atomic mass (average)
Average of all isotopes
Average atomic mass formula
E.g., Mav = (Mir-191)(%ir-191)+(Mir-193)(%ir-193)
(%ir-191) = 100% - (%ir-193)
Cations
Positive ions
Metals tend to form cations
Anion
Negative ions
Non-metals tend to form anions
Brought to you by…
Dimitri Mendeleev —
A Russian Chemist who discovered many elements and developed the modern Periodic Table of Elements
Alkali Metals
Group 1 on periodic table
Very reactive
Can explode when they come in contact with water or oxygen.
They are not found in nature on their own — they are a part of a compound
Easily lose an electron to become a positive ion.
Alkaline Earth Metals
Group 2 on the periodic table.
Semi-reactive
Also, easily lose an electron to become positive ions
Found readily in nature
Transition Metals
Group 3-12 on the periodic table
Conduct heat and electricity
Ductile — able to be deformed without losing toughness!
Malleable, bendable, flexible, etc.
Halogens
Group 17 on the periodic table
The word Halogen means salt forming
Highly reactive with the Alkaline Earth and Alkali Metals
Noble Gases
Group 18 on the periodic table
Glow when you pass an electric current through the gas!!
Doesn’t react with other elements!
That’s why it’s relatively safe to inhale helium - except you can suffocate because you’re not providing your brain with any oxygen…
Non Metals
Hydrogen, Carbon, Nitrogen, oxygen, phosphorus l, sulfur, + selenium!
These are dull, brittle, and reactive with other elements.
Technically, everything above this bolded stair-like line is a nonmetal. However, only the above seven are in the official nonmetal family
Poor Metals
Between the transitional metals and the metalloids
Just as their name suggests these have poor qualities: soft, not great conductors, and lightweight
Metalloids
Boron, silicon, germanium, arsenic, antimony, tellurium + polonium!
Conducts electricity and essential for the production of electronics and computer chips!!
AKA: the stair stepper
Lanthanides
Elements with an atomic number of 57-71
Soft, malleable, and highly conductive
Usually found as alloys (compounds with multiple metals)
Actinides
Elements with an atomic number 89-103
Only the first four (Ac, Th, Pa, & U) occur naturally
All radioactive!! — spontaneous emission of radiation (a type of wave) due to an unstable nucleus
Making a new element
Every element after uranium is synthetic (man made)
High energy particle accelerator: machines that move atomic nuclei at high speeds until they collide
How is the periodic table organized
Firstly, by number of protons
Mass too!
As you go from left to right, each element is larger than the one before it
States of matter and temperature
All elements are shown at room temperature (25 C)
Most elements (103) are solids, 11 are gases, and 2 are liquids
Metals throughout the table
Most metals are found on the left hand side and middle of the periodic table
Gasses are found on the right hand side
Valence electrons
A Valence electron is an electron in the outermost shell or energy level of the atom
The 8 major columns depict the same number of valence electrons as their column number
Note: the 8th column has full shells!
Atomic Radius
The distance from the center of an atom to the boundary within which the electrons spend 90% of their time
Can be determined using x-ray crystallography, neutron diffraction, or electron diffraction
The radius of an atom is the distance from nucleus to the outermost orbit
Atomic size increases down a group due to the increasing number of orbits occupied and a decrease in effective nuclear charge (the apparent charge experienced by valence electrons)
Atomic size decreases across a row due to increasing number of protons in the nucleus that exert a stronger effective nuclear charge on valence electrons
Ionization Energy
The amount of energy required to remove the outermost electron from an atom or ion in the gaseous state
When an atom loses an electron the remaining ion is positively charged. Energy is required to remove an electron
A(g) + energy — A+(g) + e-
After one electron is removed, it is still possible to remove more.
A+(g) + energy — A2+ + e-
More energy is required for second ionization because there are now less electrons but the same number of positive charges attracting them to the nucleus
Noble gases require the most ionization energy. They are small stable elements. Tight hold on electrons, high Zerg.
Ionization energy decreases down a group as the outer electrons are held further away from the nucleus and require less energy to be removed
Ionization energy increases across a row as the effective nuclear charge becomes larger as more protons are added but the number of orbits stays the same
Electron Affinity
The energy absorbed or released when an electron is added to a neutral atom
If a neutral atom gains electrons it may become negatively charged. In this process energy may be released, or energy may be needed to add the electron
This energy is the atom’s electron affinity — how likely it is to gain an e-
Negative values indicate energy is released
The more negative a value, the more stable the ion - more likely to gain e-
Electron affinity decreases down a group
Electron affinity increases across a period
Zeff
These trends can all be linked to electron arrangement and Effective Nuclear Charge “pull” (Zeff), which is the net positive charge experienced by an electron in a multi-electron atom
Zeff increases from left to right across a period and decreases down a group on the periodic table
Protons vs. Electrons
In a multi-electron atom, the positive charge of the nucleus is partially offset by the negative charge of the inner-shell electrons
Shielding Effect
These inner-shell electrons (core electrons) “shield” the outer electrons from the full attractive force of the positively charged nucleus
Chemical Bonding
Binding is caused by the interactions between the valence e- of atoms
Atoms tend to gain stability (ie. a lower energy state) by gaining, losing or sharing valence electrons, in order to gain a complete octet
Properties of Ionic vs. Covalent Compounds
If a metal atom is bonding with a non-metal atom in a compound, then the bond is ionic
Example: NaCl, K2SO4, MgCl2
If all the elements in the substance are non-metals, then the bond is covalent
Example: C2H5OH, C12H22O11, H2O, CO2
If all the elements in the substance are metals, then the bond is metallic
Example: alloy - bronze, steel, brass
Ionic
Soluble in H2O
Electrolytes (conduct in H2O)
Solids at room temperature
Higher fixed points (melting or boiling point)
Covalent
Varying solubility in H2O
Not electrolytes
Solid, liquid or gas at room temp
Low fixed points (melting or boiling points)
Electronegativity (EN)
Is the ability of an atom to attract electrons when bonded to another atom
Electronegativities are listed under the atomic number of your periodic table.
Across a period: EN increases due to a smaller radius and higher effective nuclear charge (“pull”)
Down a group: EN decreases due to larger size and weaker effective nuclear charge
The electronegativity difference (triangle EN) between two atoms can be calculated in order to predict the type of bond that would form between them
If the change in EN >1.7, the bond is IONIC. If the change in EN <1.7, the bond is COVALENT
The Bonding Continuum
Bonding between atoms can be classified on a continuum, which depends on the change in EN between the two atoms
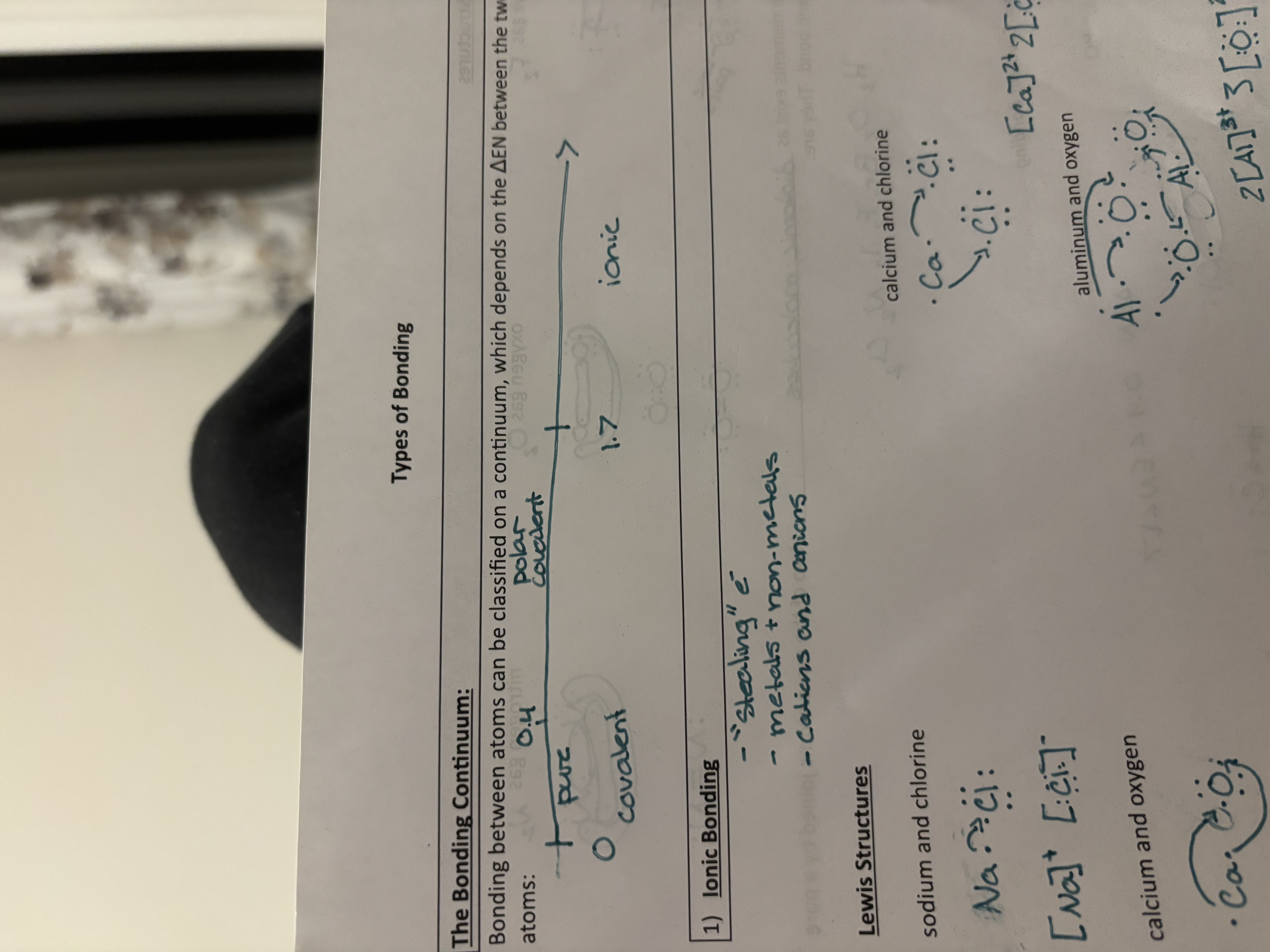
Pure covalent bonding
H2
Change in EN = 0-0.4
Covalent bond, two shared electrons
air. The electrons of both atoms spend an equal amount of time circling BOTH hydrogen nuclei
Seven elements exist as diatomic molecules- two of the same atoms joined by a pure covalent bond
Polar Covalent Bonding
HCl
Change in EN 0.4<EN<1.7
Cl is more electronegative, so it attracts electrons more than H. Thus, the chlorine “side” of the molecule is more negative and the hydrogen “side” of the molecule is more positive.
Dipole
We say that the molecule has a dipole and show it with an arrow pointing to its negative side.
What kind of substances would water be attracted to (adhere to)?
Charged object
Explain how capillary action would work in a tree, where water is drawn up from from the roots to the leaves against the force of gravity.
Cohesion - H2O sticks to itself
Polar covalent bond
A type of chemical bond where electrons are shared unevenly between two atoms, creating a partial negative charge on the more electronegative atom and a partial positive charge on the less electronegative atom
(EN diff. <1.67 and >0.40)
Nonpolar covalent bond
A chemical bond formed when two atoms share electrons equally because they have very similar or identical electronegativity
(EN difference < or equal 0.40)
Dipole-dipole forces
Each molecule is known as a dipole. The attraction between the positive end of one dipole and the negative end of another is called dipole-dipole force
Dipole-dipole forces arise between polar molecules
Dipole induced dipole force
When a polar molecule approaches a Nonpolar molecule, the electron cloud of the Nonpolar molecule may become distorted, causing the Nonpolar molecule to become temporarily polar. For example, if the negative end of the polar molecule approaches the Nonpolar molecule, the electron cloud of the Nonpolar molecule will be repelled, causing a slight positive charge at that end of the Nonpolar molecule. The resulting attractive force is called a dipole-induced dipole force
Dipole-induced dipole forces arise between polar and Nonpolar molecules
London dispersion forces
Even when the molecules are Nonpolar, random variations in the distribution of electrons can cause parts of these molecules to become slightly charged. This imbalance leads to very tiny, short-lived attractions between molecules called London dispersion forces
London dispersion forces arise between Nonpolar molecules
Polar molecule
Has an uneven distribution of electron density, resulting in one end of the molecule being slightly positive and the other end being slightly negative
Nonpolar molecule
Has an even distribution of electrons density, resulting in no separation of positive and negative charges and a net dipole moment of zero
(Symmetrical)