3.8 - Nuclear physics
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
Describe the plum pudding model?
The plum pudding model suggested that an atom is a sea ball of positive charge with negative electrons in embedded in it.
JJ Thompson
Describe Rutherford's alpha-scattering experiment.
- directed a beam of a-particles at a thin gold foil held within a vacuum
- they counted the number of a-particles that were deflected by the gold leaf through different angles
equipment:
- lead collimator: produce the narrow beam
- gold foil target: can be rolled to a sheet only a few atoms thick
- chamber is evacuated
- zinc fluoride fluorescent screen: allow the viewer to see when a-particles meet the screen
- rotatable detector: allows the angle of a scattering to be determined
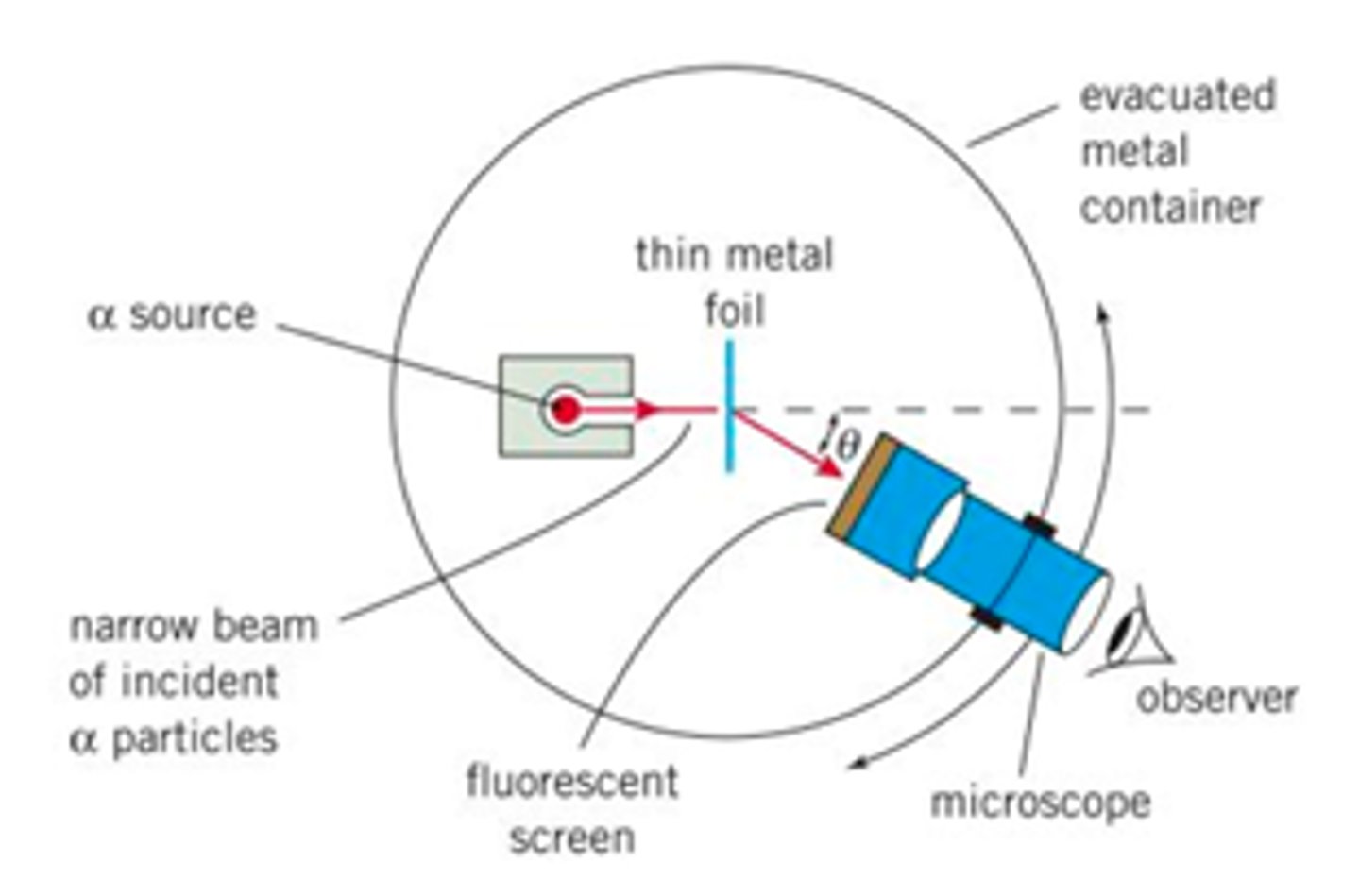
Why was the chamber in Rutherford's experiment evacuated?
a particles can only penetrate 3-5cm in air. they would be absorbed by the air without the vacuum.
What were the results of the a-scattering experiment?
- most passed straight through undetected
- a few particles are scattered through small angles
- tiny proportion of a particles were backscattered (angle greater than 90)
What did Rutherford expect to be the results?
absorbed or passed straight through
Explain the results of Rutherford's experiment.
- electrostatic force causes the backscattering
- it is proportional to the product of the two charges and inversely proprtional to the square of the distance between them
- for there to be sufficient force to cause the observed backscattering, all the charge must be concentrated at the centre of the atom
- if we consider conservation of momentum, the change in momentum of the a-particle when it backscatters implies that it has collided with something more massive than itself therefore the mass of the atom must be concentrated in the centre
- only the a-particles that pass closest to nuclei are deflected; most pass through undeflected. This means most of the atom is empty space.
Descibe a film badge.
The badge has 6 filters.
1. open window
2. thin plastic
3. thick plastic
4. dural filter
5. tin/ lead filter
6. cadmium filter
These different filers allow the amount of exposure to each form of ionising radiation to be estimated from the level of blackening of each part of the photographic film.
Describe safe handling of radioactive sources in a school laboratory.
- the distance between the source and the user is increased by using tongs
- the sources must always be pointed away from people
- the exposure time must be minimised
- the sources must be kept in a lead-lined box when not in use
Name some uses of radioactivity.
smoke detector
measuring the thickness of various materials
sterilising in medical experiments
tracers in both medicine and industrial applications
radiotherapy
dating rocks/ carbon dating
Describe how smoke detectors work.
Alpha particles ionise the air between two charged plates. While the air is ionised, a current flows.
Smoke particles neutralise the ions so the current stops. This sets ofd the alarm.
Why is alpha radiation used in smoke detectors?
- its is highly ionising
- only has a short range in air
The half life of the source should be long so that the source does not need to be continuously replaced.
Describe radioactivity and sterilisation.
- gamma rays are used to kill bacteria, mould and insects in food
- this can be done even after the food has been packaged
(it can affect the taste, but also lengthens shelf life)
- gamma rays are also used to kill bacteria on hospital equipment
- it is particularly useful with palstic equipment that would be damaged by heat sterilisation
Describe radioactive tracers used in leak detection in pipes.
The radioactive isotope is injected into the pipe.
Areas of high radioactivity outside the pipe indicate where the pipe is leaking.
Useful for underground pipes that are hard to access.
The radioisotope must be a gamma emitter so it can be detected through the metal and earth where the pipe leaks. Alpha and beta rays would be blocked. The isotope must have a short half life so that the material does not become a long term problem.
Describe sources of background radiation.
rocks in the ground, radon gas in the atmosphere, cosmic rays
Why is ionising dangerous inside the body?
it damages and kills cells
How is ionising radiation detected?
a geiger tube
What is the formula for electron capture?
P + e- → n + electron neutrino
What does penetrating power depend on?
the initial energy of the radiation
the density of the material it is passing through
the size of the particle (more collisions per unit distance, more ionising, less penetrating power)
How do we account for background radiation in experiments?
before starting the investigation, count rate without the source present must be meaured
this value will later be subtracted from subsequent measurements
Describe the range of alpha particles in air.
They have a range of only a few cm beyond which the count rate drops sharply. The range of the particls is the same for a-particles from a particular source but can change from source to source. This sugests that the inital kinetic energy of the a-particles is the same for each source, but differs between sources.
Describe use of radiation in medicine.
- a radioactive isotope can be administered ot a patient and then its lcoation traced using a camnera outside the patient. It must be a gamma source
- a controlled beam of gamma rays can be used to kill cancer cells. It must be directed carefully to minimise the famage to normal cells
- However, some damage is unavoidable and this can make the patient ill.
- There is a balancing act; getting the dose high enough to kill the cancerous cells, but as low as possible to minimise the harm to the patient.
Describe the range of B-particles in air.
They have a range in air of up to about 1m. The count rate gradually ecreases with distance, showing that the B particles from a particlar source havbe a range of initial kinetic energies.
Describ eht rane of gamma rays in air.
It has an unlimited range in air, but the count rate decreases with distance due to the spreading out of the radiation in all direction. This decrease is in accordance with the inverse square law.
What is the inverse square law?
intensity, I = k/(r^2)
What is the constant k in the inverse quare law?
nhf/4pi
Describe deflection of alpha and beta particles in magnetic and electric fields.
They are deflected in opposite directions. B are deflected more than a because even though the charge is half the magnitude, the mass is around 8000 times smaller
How and why are gamma rays deflected in electric/magnetic fields?
they are undeflected in both because they carry no charge.
Define radioactive decay.
the random process by which an unstable nucleus spontaneously decays and emits a particle of radiation
What is the decay constant? Units?
the probability that a particular nucleus will decay in the next second.
s^-1 (per second)
lamda is the symbol λ
Defien activity.
the activity, A, of a sample is defined as the number of decays per second
What is the formula for activity?
A = λN
N = the number of nuclei in the sample
Define half life.
The half-life is the time taken for half the number of nuclei in a sample of unstable nuclei to decay.
What are typical values for nuclear radius?
hydrogen: 2 fm
carbon-12: 3 fm
Which methods can we use to estimate nuclear radius?
- electron diffraction
- distance of least approach
- a-scattering using past data.
How can we use distance of least approach as an estimate for the nuclear radius?
As an a-particles appraoches a nucleus, the energy in its kinetic store is tranferred to the electrostatic potential store. In a head-on collision, Ek=0 at the closest point to the nucleus, so initial Ek = Ep at the least distane of approach.
From couloumb's law, Ek = Q1Q2/(4piε0d)
We can use the distance of least approach as an estimate.
How can we use a-scattering using past data to estimate for the nuclear radius?
In Rutherford's experiment, 1 in 10^4 a particles where deflected by more than 90º. So d = D/10^4, or the nucleus has a diameter about 10,000 times smaller than an atom.
How can we use electron diffraction to meaure to nucleus?
Electron diffraction by a crystalline structure shows a pattern of concentric rings, when the de Broglie wavelength of the electron is similar in size to the interatomic spacing (10^-10).
Diffraction patterns can also be produced by scattering electrons off the nuclei inside atoms, but as nuclei are 10000x smaller than atoms, the de Broglie wavelength needs to be correspondingly smaller. (10^-14 or 10^-15)
As high energy electrons have speed close to that of light, we use E=mc^2 in the de Broglie equation so we get λ=hc/E
From this we can find the energy of the electrons required to have a wavelength of the same size as a nucleus of diameter ~10^-15
What are the advantages of using electron scattering?
- unlike a particles, electrons do not experience the strong nuclear force so the only interaction that is involved is electromagnetic
- with a particles, it is the closest distance of approach that is measured, rather than the nuclear radius which gives an overestimate of the size of the nucleus
- electrons cause far less recoil of nuclei than a particles because they have a much smaller mass
- electrons give greater resolutiom
What is the formula for nuclear radius?
R = r0A^1/3
R0 = a constant (size of an individual nucleon)
A = mass number
How can the relationship between R and A be found?
plotting a grpah of ln A against lnR
How can nuclear density be linked to the strong nuclear force?
This constant nuclear density implies that the strong force acts on all nucleons. It must have a very short range.
It implies that, since there is repulsion at very small distances. since the nucleons are not being "squashed" and creating denser nuclei.
Draw the N Z graph.
- add scales up to N=120 and Z=80
- draw a line for N=Z
- the line of stability follows the line up to Z=20, it then curves upwards
- the line should go through 80,120
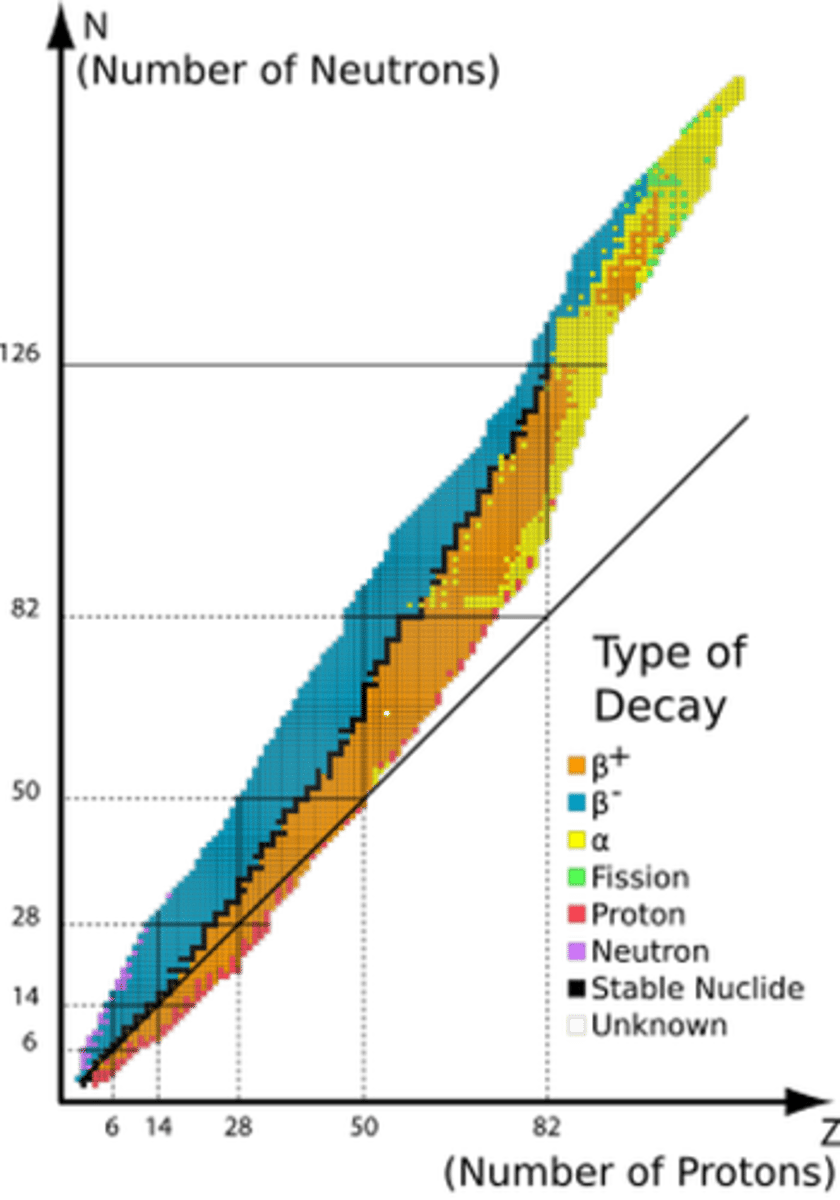
Describe how differently sized nuclei decay.
- nuclei above the line of stability have too many neutrons so decay by beta minus
- nuclei below the line of stability have too many protons so decay by beta plus
- the nuclei below the line may also decay by electron capture because this also results in conversion of a proton to a neutron
- nuclei with Z>80 and N>120 decay by alpha decay as they are too massive to be stable due to the short range of the nuclear force
Why does the N:Z ratio increase with Z?
- EM repulsion exists between all protons
- as the number of protons increases, more neutrons are needed to increase the attraction of the strong nuclear force without increasing the EM repulsion in order for the nucleus to be stable
Give an example of the use of gamma ray emission.
Some radioactive isotopes form in an excited state that have long enough half lives to be separated from their parent isotopes. Such a long lived excited state is called a metastable state. One example of this is technetium-99m which decays by gamma emission to its ground state with a half life of six hours the ground state of this nuclide is a beta minus emitter with a half life of 500,000 years which makes it suitable for use as a radioactive tracer.
What is the value of u in MeV (it is VERY helpful to remember this).
931.5MeV
Define binding energy.
The work that must be done to separate a nucleus into its constituent neutrons and protons
Define mass difference of a nucleus.
The difference between the mass of the separated nucleons and the mass of the nucleus.
Which is the most stable element?
Fe-56
How do elements above and below iron release energy?
Below; nuclear fusion
Above: fission
Define nuclear fusion.
Small nuclei fuse together to form a larger more stable nucleus.
When does nuclear fusion release energy?
When the resulting larger nucleus has more binding energy per nucleon that the smaller nuclei which have fused.
Define nuclear fission.
A large unstable nucleus splots into two or more stable nuclei.
When can nuclear fission release energy?
it can release energy for nuclei with mass numbers greater than 56. As with fusion, the bidning energy icnreases,
How can the total energy released during nuclear fission be calculated?
binding energy per nucleon x number of nucleons
What is induced fission?
when the splitting of a large nucleus into two daughter nuclei occurs due to bombardment of the alrge nucleus with thermal neutrons.
What are thermal neutrons?
slow moving neutrons that have a similar KE to room temperature gas molecules.
What are the products of induced fission?
fission fragments, 2 or 3 free neutrons and gamma photons
Describe the process of induced fission.
1. U-235 bombarded with thermal neutrons
2. neutron absorbed by U-235 nucleus to produce a U-236 nucleus which is very unstable
3. U-236 splits to form fission fragments, 2 or 3 free neutrons and gamma photons
4. if the free moving neutrons are absorbed by further U-235 nuclei, a self-sustaining chain reaction can occur.
What is the critical mass of a chain reaction?
the minimum mass at which a chain reaction will occur.
Why do chain reactions have a critical mass.
- the total number of nuclei available for fission is proportional to the volume and the number of neutrons escaping the uranium is proportional to the surface area of the sample.
- as the mass decreases, the volume will increase (as density is constant, so the surface area to volume ratio decreases, meaning a smaller fraction of the neutrons produced will escape
- this means that a larger fraction of the neutrons produced in one fssion can go on to produce more fission
How is energy released in a fission reaction?
kinetic energy of the fission fragments and the neutrons, and the emitted gamma radiation
How can you calculate the energy released in fission?
the change in binding energy results in a loss of mass; use this to calculate energy released
What is contained in the reactor core? And outside?
inside:
- fuel rods
- control rods
- moderator
- coolant
Describe the fuel rods. You must know what they are made from.
They are made from enriched uranium. This is because normal uranium is 99% U-238 which does not fission. It must be enriched to contain about 2-3% U-235, which is fissionable.
Describe the control rods. You must know what they are made from.
They are normally made from boron or cadmium.
They are used to absorb the free neutrons released to control the rate of fission.
They can be raised or lowered to keep the number of neutrons constant so that one neutron from each fission is able to induce another fission.
Describe the moderator. You must know what it is made from.
- the neutrons released travel too quickly to idnuce another fission
- the moderator slows them down
- it is made from water or graphite
how does the moderator slow down free neutrons?
through repeated elastic collisions with the atoms inside the moderator
Describe the coolant.
- it is used to take heat from the reactor core to the heat exchanger
- water is normally used, but CO2 or helium gas can also be used
- in a pressurised water reactor (PWR), the mdoerator used is the same water that is used as the coolant. It is pressurised so that the water remains in the liquid state at the high temperatures found in the reactor core.
Describe the heat exchanger.
It is used to transfer the heat from the coolant to water that is turned to steam to drive a turbine and generator.
Why is gamma a good choice for sterilisation of medical instruments?
it has a high penetrating power so can irradiate all sides of the medical instruments
Why doesn't gamma radiation cause nuclei to become radioactive?
ionising radiation does not affect the nucleus
What are the advantages of nuclear power?
- some nuclear power stations can adjust their outputs quickly
- consistent source of energy
- little air pollution
- very little CO2 produced; small contribution to global warming
Describe safe handling of radioactive sources in industry and medicine.
- users of equipment that produces ionising radiation must wear a film badge to monitor their exposure
- their exposure time is minimised
- they may wear protective clothing