chem161, practicetest1 review
1/5
Earn XP
Description and Tags
review on the topics you were struggling with
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
What electrons are valence electrons?
Its the highest level
Cool trick —>
there are numbers on the top of the periodic table….
if element is in the main group the number of valence electrons is the number on top
if the element is in the transition group the number of valence electrons is the number is 2 + how many electrons are in the lower d orbital
When we remove electrons from an electron do we take from the right orbital
(when in 1s22s22p….. form) or do we take from the highest n?
W
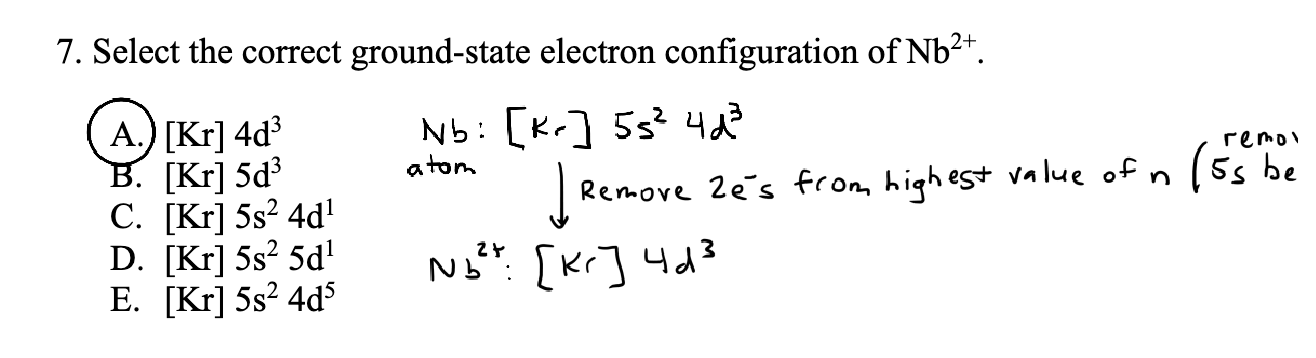
e take from the highest n..
when you are doing equations should you double check them on the sheet??
… yes
Can moles represent anything other than atoms
Yes, you can have 6.022 x 10²³ (Avogadro's number) moles of anything because a mole is a unit that counts a specific number of entities, similar to a "dozen" counting 12 things. This number can represent 6.022 x 10²³ atoms, molecules, ions, or even other discrete objects, though it is most useful in chemistry for counting particles like atoms and molecules

How should you solve this question?
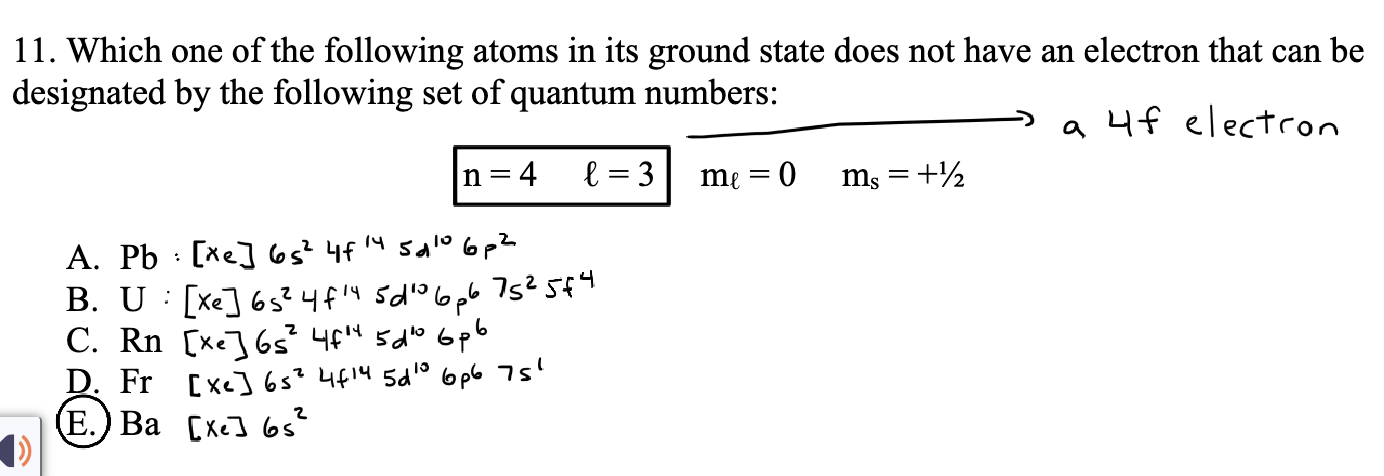

also give me the notation..
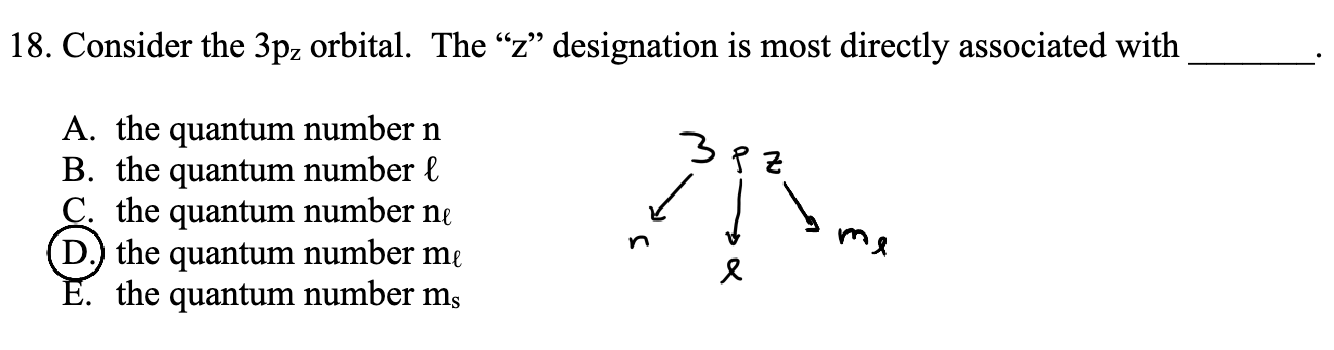
The number (n) (for example, 3) indicates the energy level or shell.
The letter(L) (s, p, d, f) tells you the shape of the orbital.
The subscript(ML) (1, -1, or 0) tells you the orientation of the orbital in space.
If there’s an arrow in a box diagram, it shows the electron’s spin(ML) (up or down)