M7 Baena- Life requires a continual energy flux
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
how does the organisation of life not defy the second law of thermodynamics?
the second law: the total entropy of a system and its surrounding increases for a spontaneous process
life seems to disobey this because small molecules spontaneously assemble into a highly organised state (decrease in entropy)
however, local entropy can spontaneously decrease as long as the total entropy of the system increases
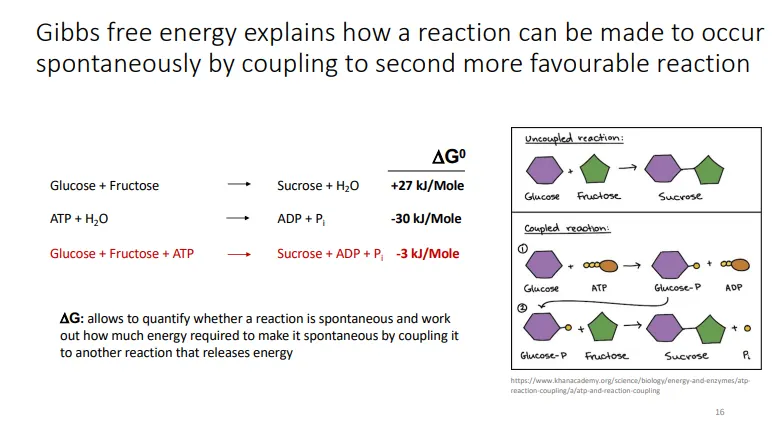
life is possible because its biochemical processes:
increase the entropy of the environment by taking up highly ordered forms of energy and releasing less ordered forms (and using the energy released in catabolism to produce ATP)
couple non-spontaneous reactions with more energetically favourable ones (primarily the hydrolysis of ATP)
the energy released can be used to produce macromolecules and decrease the entropy within the cell
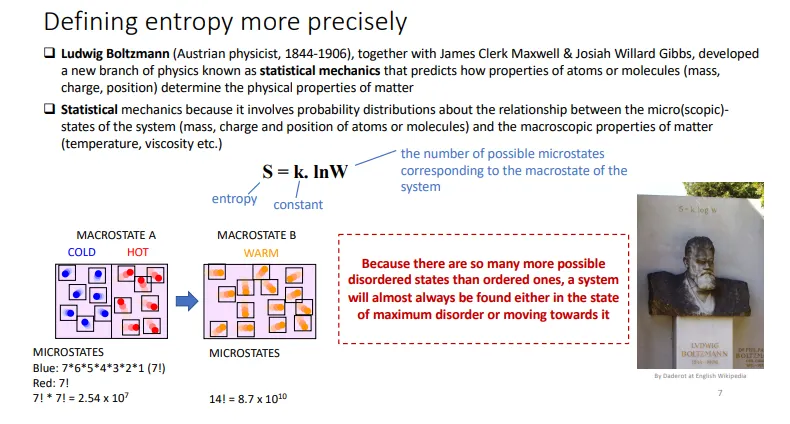
what is entropy?
entropy is a measure of disorder in a system
S = k x lnW, where:
S = entropy, k = constant and W = the number of possible microstates for the macrostate of the system
why is ATP the universal energy currency?
can be produced from adenine nucleotides
adenine is the only base that doesn’t include oxygen (so can be produced easily in anaerobic conditions)
m
k