Preliminary Chemistry Modules 1-4
1/120
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
121 Terms
Mixtures
A mixture is a combination of two or more pure substances (containing one type of molecule) - the mixture itself is impure.
Heterogeneous Mixtures
Two or more substances intermingle but remain physically separate.
E.g dirt and sand, oil and water, salt and baking soda
A suspension is a specific type of heterogeneous mixture where particles settle at the bottom.
Homogeneous mixture
Two or more substances have merged into a uniform phase. There are no borders between the substances, but they are not chemically bonded. The physical properties of each ingredient can be exploited to separate them.
E.g. Saltwater, Copper Sulfate solution, aqueous solutions
Elements vs Compounds
Elements are pure substances that cannot be chemically or physically decomposed.
Compounds are pure substances that are chemical combinations of two or more different elements - they can be decomposed.
Periods
The rows of the periodic table. Each period corresponds to the number of electron shells of that elements.
Groups
The columns of the periodic table. Elements in the same group share similar chemical properties, as they have the same number of valence electrons.
Periodic properties
Atomic Radius, Ionisation Energy and Electronegativity
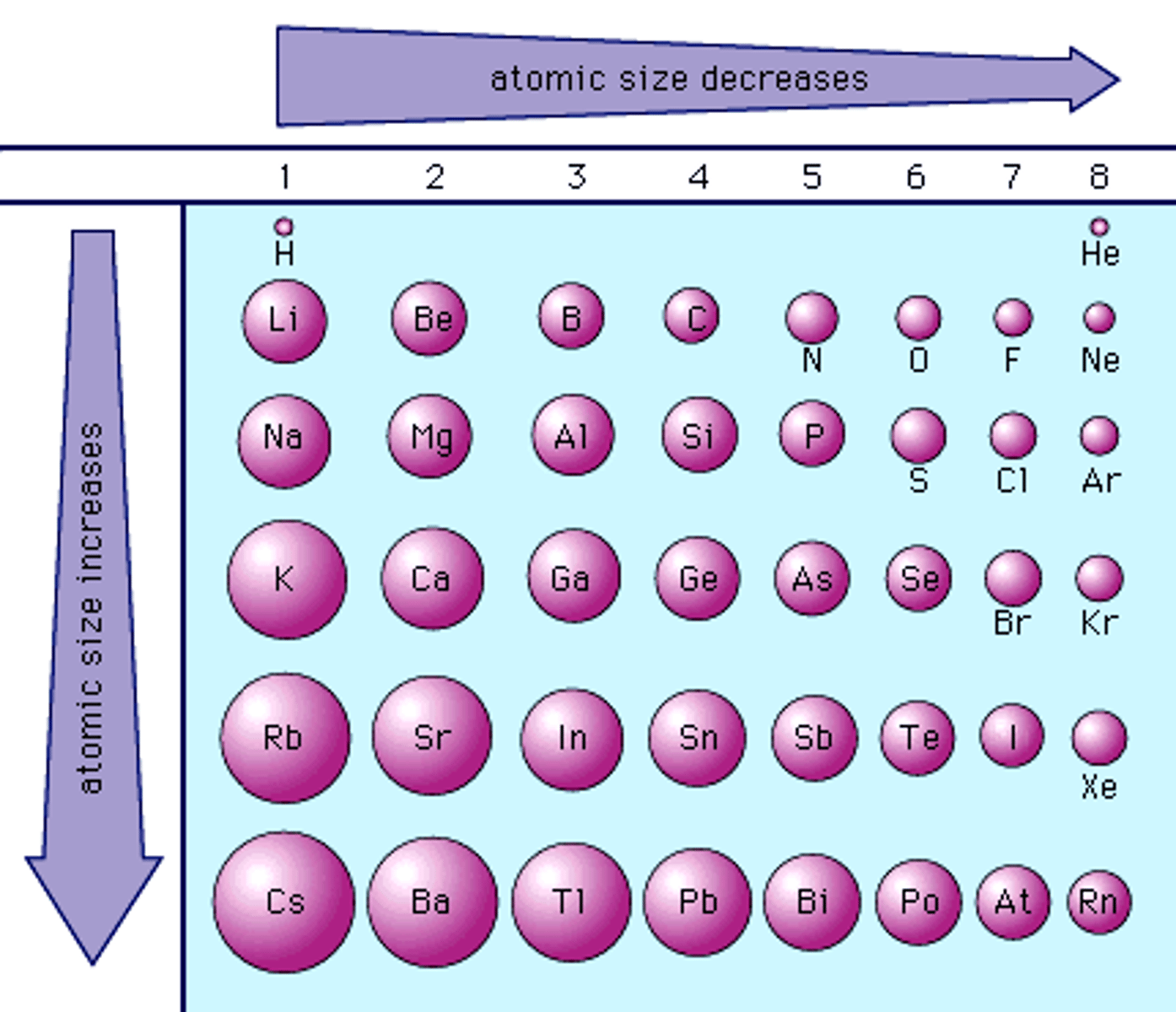
Atomic Radius
Half the distance between the centers of two atoms of an element that are touching
Going left → right across a period, atoms have more protons but the same amount of electron shells. Thus, Electrons are attracted to the nucleus more strongly, and the atomic radius decreases
Going up → down the group, atoms have more electron shells, which not only put the valence electrons further away, but the inner electrons also repel (or shield) the valence electrons from the nucleus's attraction, so the atomic radius increases
Atomic radius affects all the other properties - i.e. it's easier for an atom with a greater atomic radius to let go of an electron, as greater ionisation energy
Ionisation Energy
The energy required to remove one valence electron from a gaseous atom.
The more strongly bound to the nucleus electrons are, the more ionisation energy is required to remove them
Smaller atomic radii mean stronger bound electrons, so ionisation energy increases as atomic radius decreases
A low first ionisation energy indicates that an element is a metal, while a high first ionisation energy indicates that it is a nonmetal
Electronegativity
The measure of the ability of an atom to attract electrons for chemical bonding (measured in Pauling units).
When an atom has a smaller atomic radius and the atom can easily pull external electrons into it. Thus, as atomic radius decreases, electronegativity increases
FON = most electronegative
Periodic Trends
Moving Left → Right (Periods):
Ionisation Energy Increases
Electronegativity Increases
Atomic Radius Decreases
Moving Up → Down (Groups):
Ionisation Energy Decreases
Electronegativity Decreases
Atomic Radius Increases
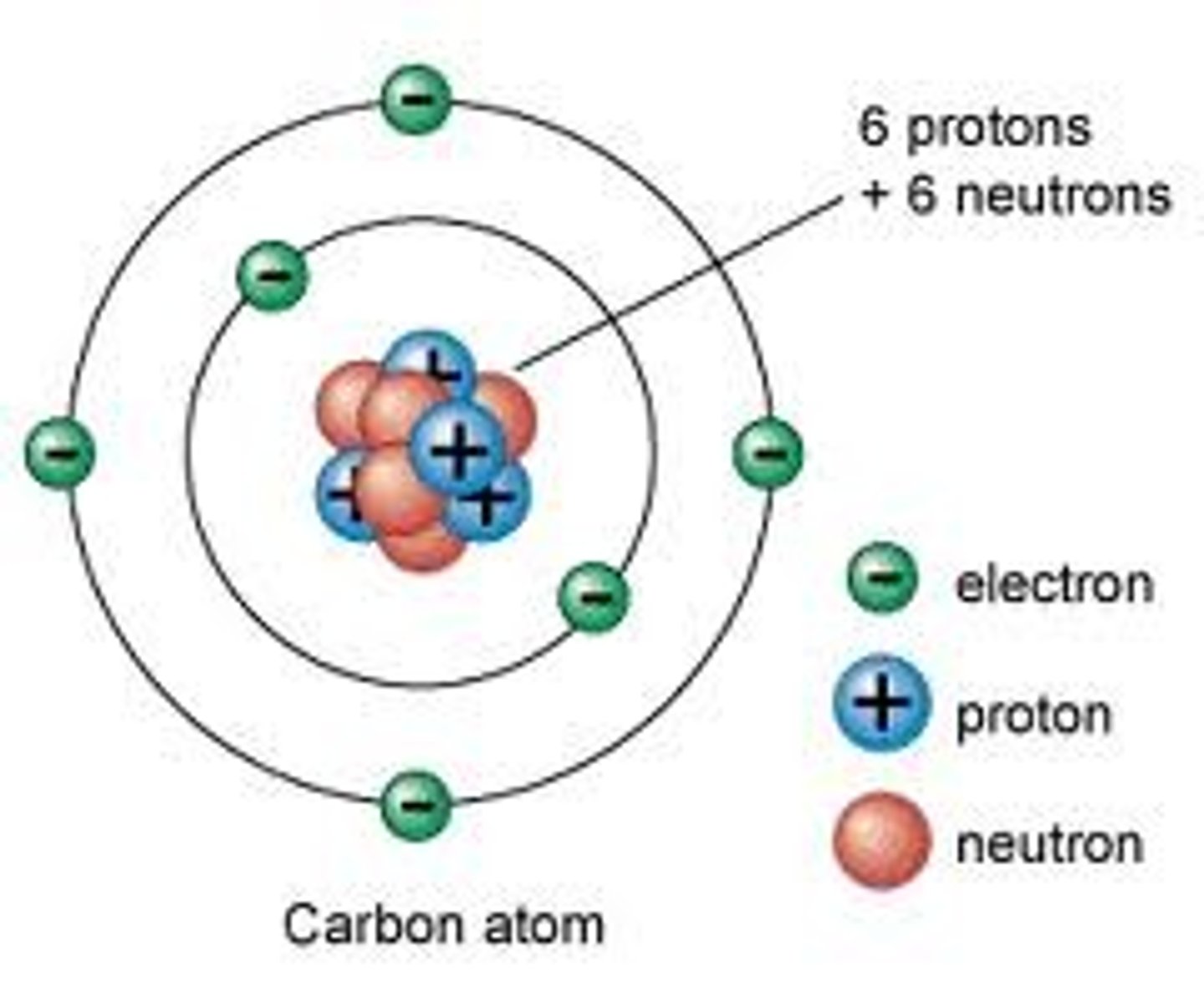
Isotopes
Elements with the same number of protons and electrons but a different number of neutrons.
e.g. a Hydrogen atom can have 0, 1 or 2 neutrons, but it is still hydrogen.
Isotope Trends
Isotopes with atomic number > 82 are all unstable.
Isotopes with 1:1 proton-neutron ratio are much more likely to be stable.
Atoms with an even number of protons and neutrons are more likely to be stable.
Mass spectrometer
Device that uses electromagnetic fields to sort the isotopes present in a substance by atomic mass, which then allows us to see how abundant each isotope is.
Half life
Measure of the time it takes for half the atoms in that substance to decay. Half-lives can range from seconds to billions of years, and can be represented as a logarithmic graph.
Types of radiation
Alpha, Beta, Gamma
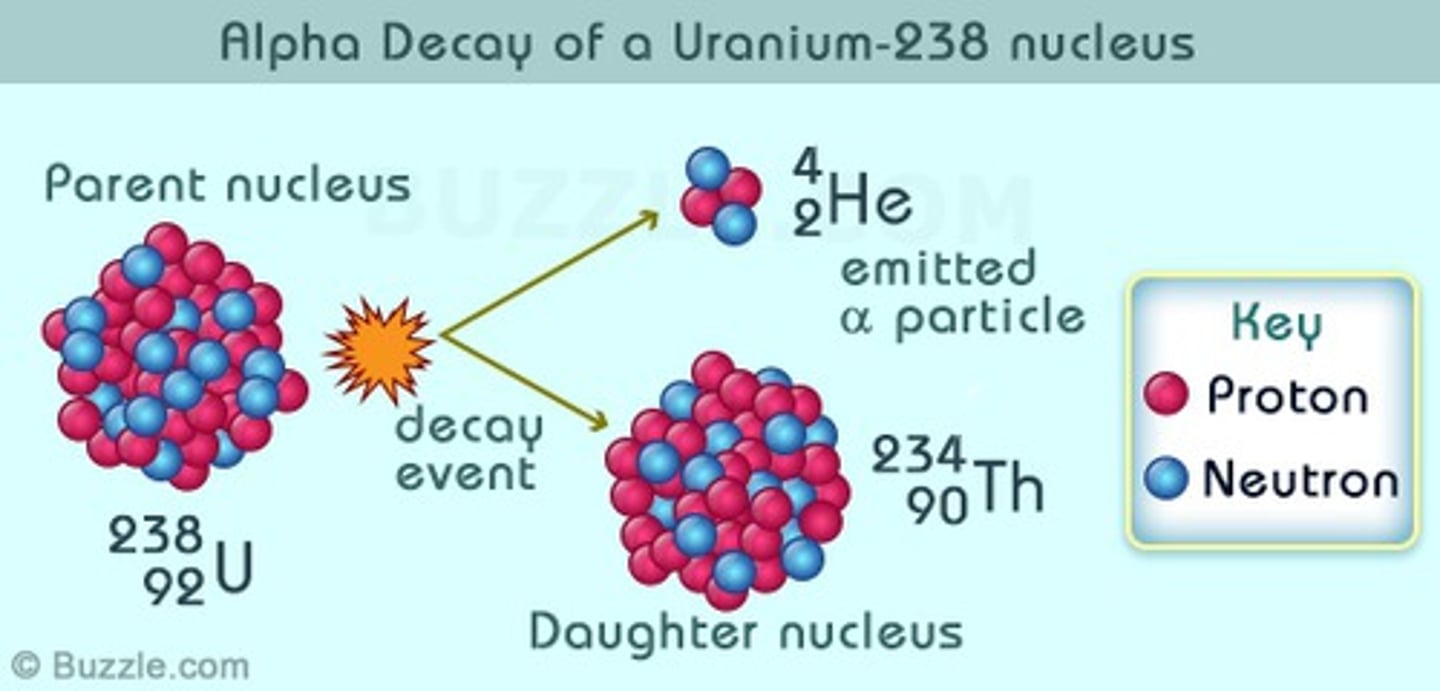
Alpha Decay
Occurs when an atoms mass is too much therefore emits an alpha particle a Helium particle (2 protons, 2 neutrons)
Atomic number decreased by 2, mass decreased by 4.
For example, uranium-238 transforms into thorium-234.
Beta Decay
Occurs when an atom has too many neutrons therefore emits a beta particle - proton changes into a neutron and a positron; the positron is emitted.
Atomic number decreases by 1, mass is conserved.
For example, thorium-234 (90) becomes protactinium-324 (91)
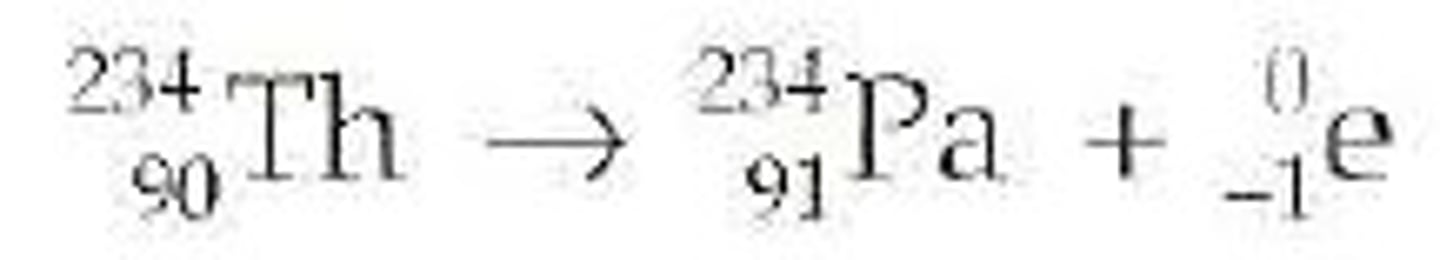
Gamma Radiation
Occurs when an atom emits gamma rays. It usually occurs after alpha or beta decay, where the nucleus is still excited after decaying. The excited nucleus then releases gamma ray photons to become more stable. Gamma Radiation technically isn't a type of decay because only energy is released.
Penetrative power of each radiation
Alpha: Few centimetres in air
Beta: Few millimetres of aluminium
Gamma: Many centimetres of lead
Bohr Model
1. Electrons are particles that occupy fixed orbits around the nucleus - called stationary orbits
2. Each orbit has an energy level associated with it
3. Energy is absorbed when an electron jumps from a lower orbit to a higher one and energy is emitted when an electron falls from a higher to a lower orbit
4. Electrons cannot exist between the energy levels/orbits
Energy Levels and Electron Configuration
Electrons do not orbit the nucleus in fixed orbits, they move around in energy levels called
orbitals - which are regions around the atom where the electron is very likely to be found at any given time.
Aufbau Principle
States that in order to configure electrons in an atom, electrons are added to the lowest energy level until it is filled. Then electrons are added to the next energy level until that is filled, etc.
spdf notation
The principal shells are named in increasing order of energy as 1,2,3, etc, while the subshells are named s, p, d, f. Each subshell can hold a maximum amount of electrons, depending on the orbitals that it contains.
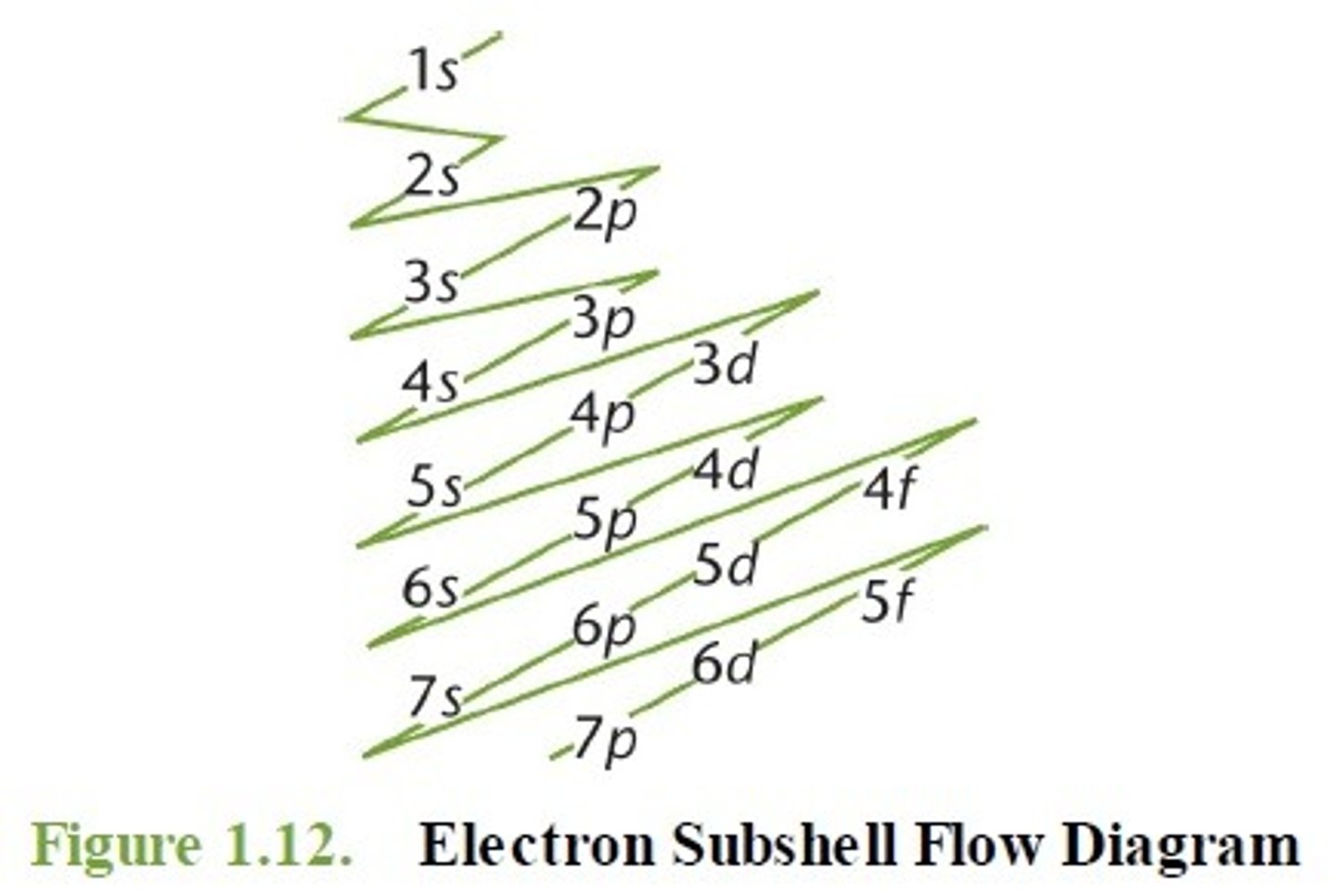
For example titanium which has 22 electrons
1st diagonal: 1s (add 2 electrons) → 20 electrons left
2nd diagonal: 2s (2 electrons) → 18 electrons left
3rd diagonal: 2p (6 electrons), 3s (2 electrons) → 10 electrons left
4th diagonal: 3p (6 electrons), 4s (2 electrons) → 2 electrons left
5th diagonal: 3d (can hold 10 electrons, but add 2) → 0 electrons left
1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 3d2 , 4s2
Bonding
In order to gain a full shell or noble gas configuration, atoms will remove/gain electrons to make itself more stable. Electronegativity is the main mechanism behind bonding.
Ionic Bonds
Involve a Metal donating electrons to a Nonmetal atom.
The metal atom becomes cation) (+) after losing electrons while nonmetal atom becomes anion (-) after gaining electrons, creating ionic compound.
E.g. sodium chloride where sodium (cation) and chlorine (anion).
Covalent Bonds
Involve a nonmetal sharing electrons with another nonmetal atom. They bond as these electrons are shared to form a covalent or molecular compound.
E.g. Hydrogen bonds with Oxygen, two hydrogen atoms each share an electron with an Oxygen atom and form Water (H2O)
Polarity of Covalent Bonds
Electrons can be shared equally in covalent bonds, however not often. Occurs when one atom has higher electronegativity (wants the electrons more).
Often occur between atoms that are the same, such as two Cl atoms or H atoms.
In Polar Covalent Bonds, electrons NOT shared equally between the atoms, because the electronegativity of the atoms is different.
Atom with higher electronegativity = slightly negatively charged (δ−)
Atom with lower electronegativity = slightly positively charged ( δ+).
Bond is partially ionic.
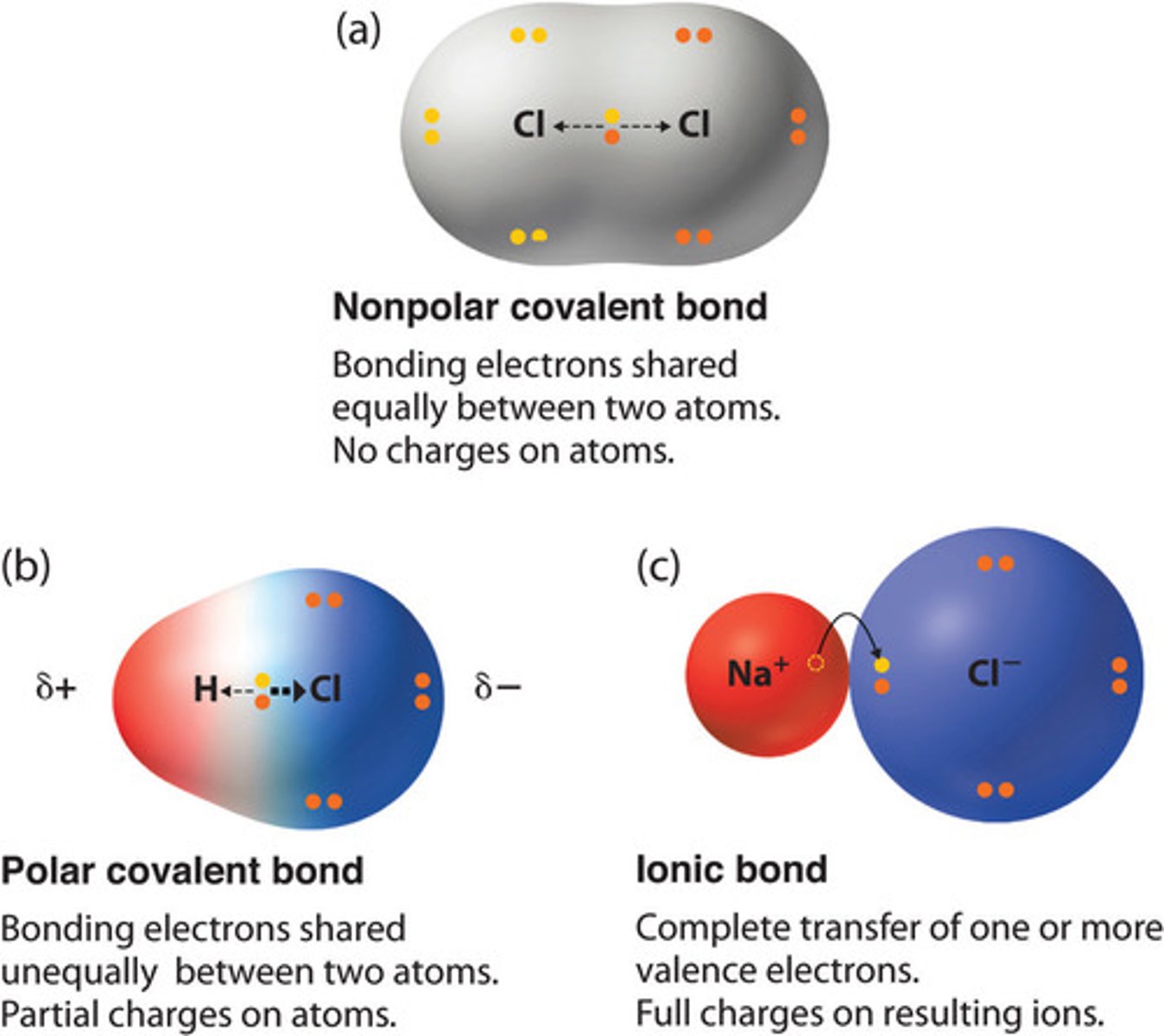
The higher the electronegativity difference, the more _______ the bond is
ionic
The electronegativity of an element can generally be predicted by its periodicity - so two elements such as Hydrogen and Bromine which are located far from each other would make a very polar covalent bond.
Lewis Dot Diagram example
Carbon dioxide
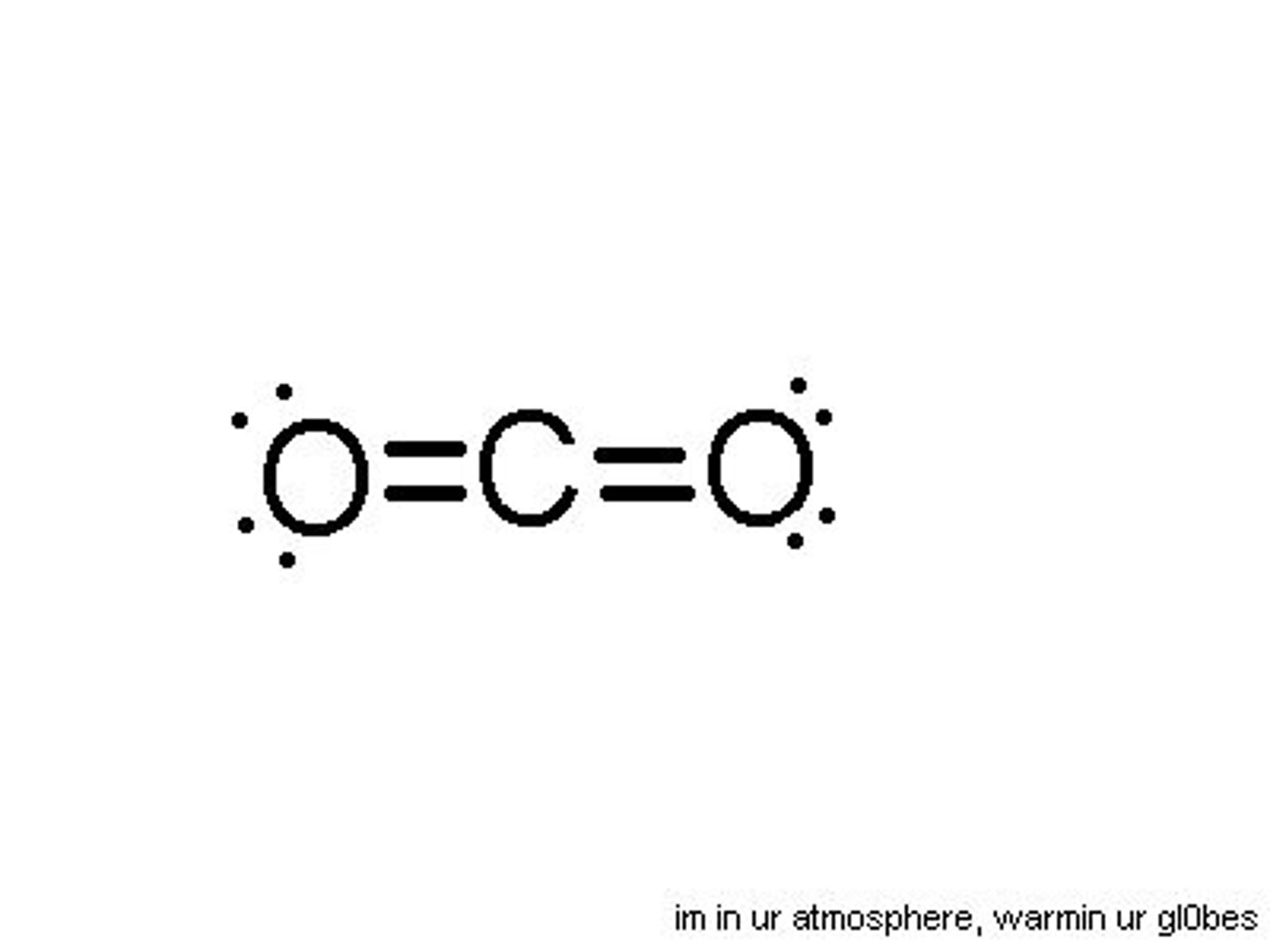
Intermolecular forces
Are the forces that exist between molecules. These forces can also arise from electromagnetic attractions holding molecules together.
Intra vs Intermolecular forces
Intramolecular bonds are much stronger than intermolecular forces - breaking apart the atoms inside a molecule requires a lot more energy than breaking molecules away from each other.
The larger atomic radius, the ... its intermolecular forces
The more electrons an atom has (i.e larger atomic radius), the stronger it's intermolecular forces.
If it is a solid, it has stronger intermolecular forces than a substance that is a gas at room temp.
Dipole-Dipole Interactions
When multiple molecules are in polar covalent or ionic bonds, one side of the molecule is slightly positive, and one side is slightly negative. These sides are known as dipoles. When the negative dipole of one molecule attracts to the positive dipole of another, they bond. This is the strongest intermolecular force.
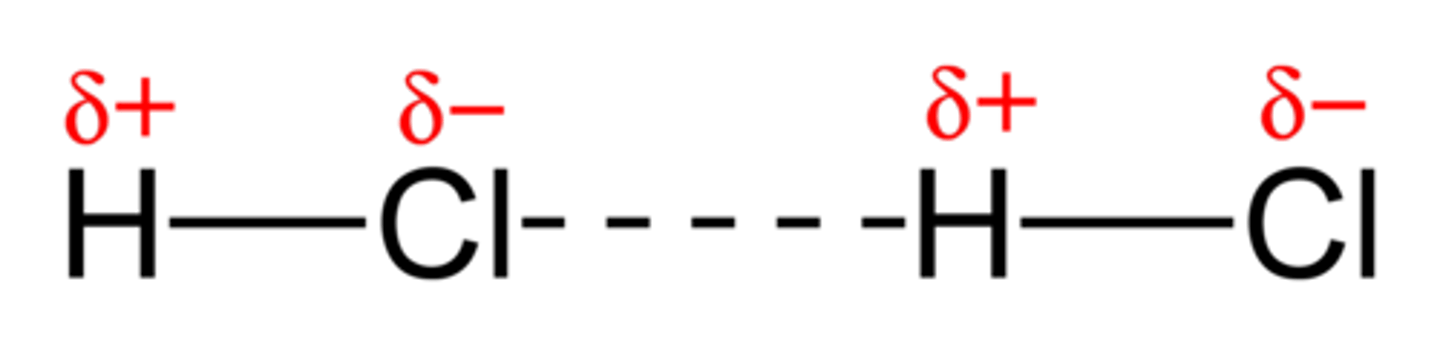
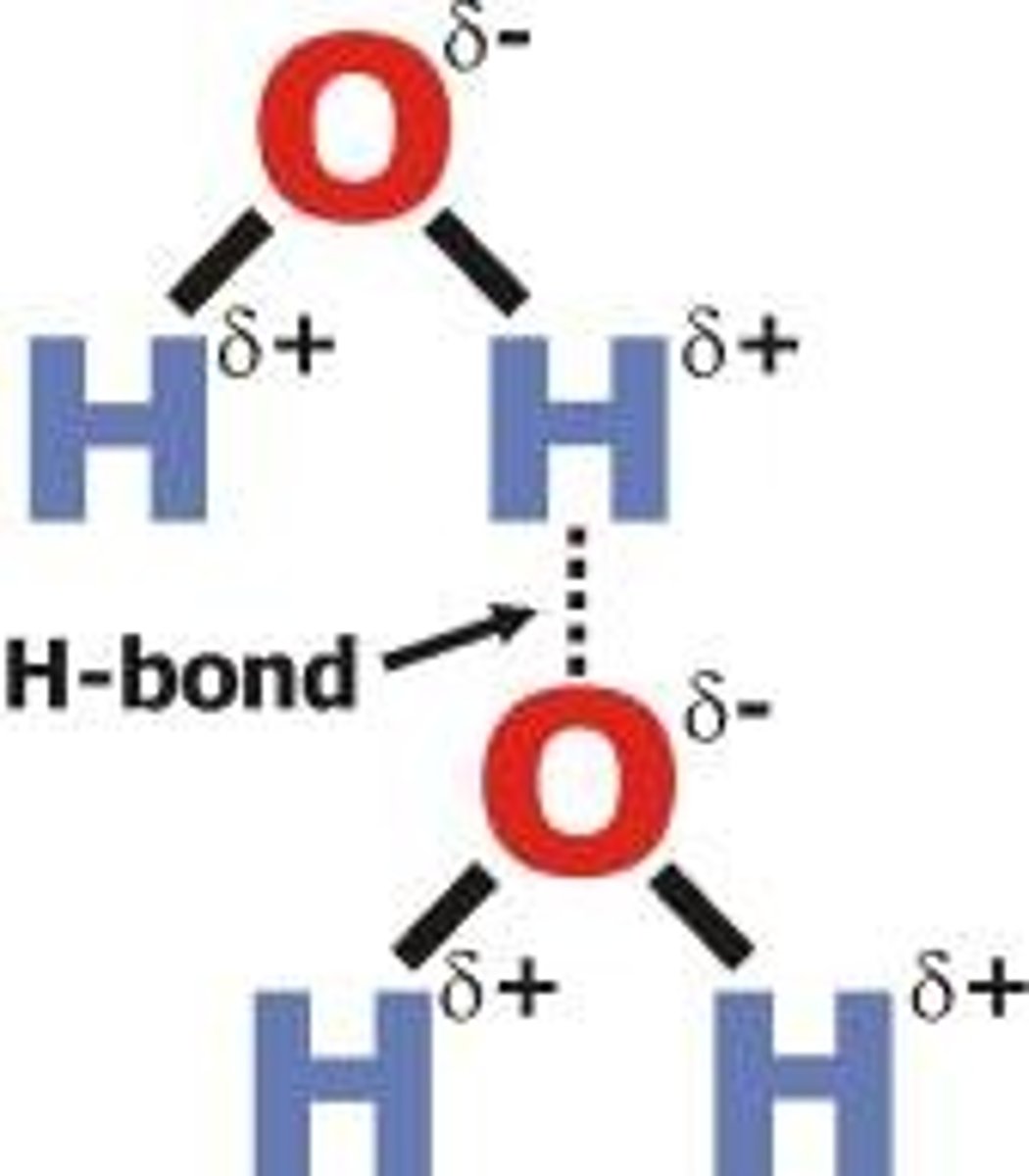
Hydrogen Bonds
a special kind of dipole-dipole interaction that occurs between hydrogen atoms bonded to either FON atoms. The positive dipole of Hydrogen is attracted to the negative dipole of fluorine/oxygen/nitrogen. Due to the large electronegativity difference, this is the strongest version of dipole-dipole interactions.
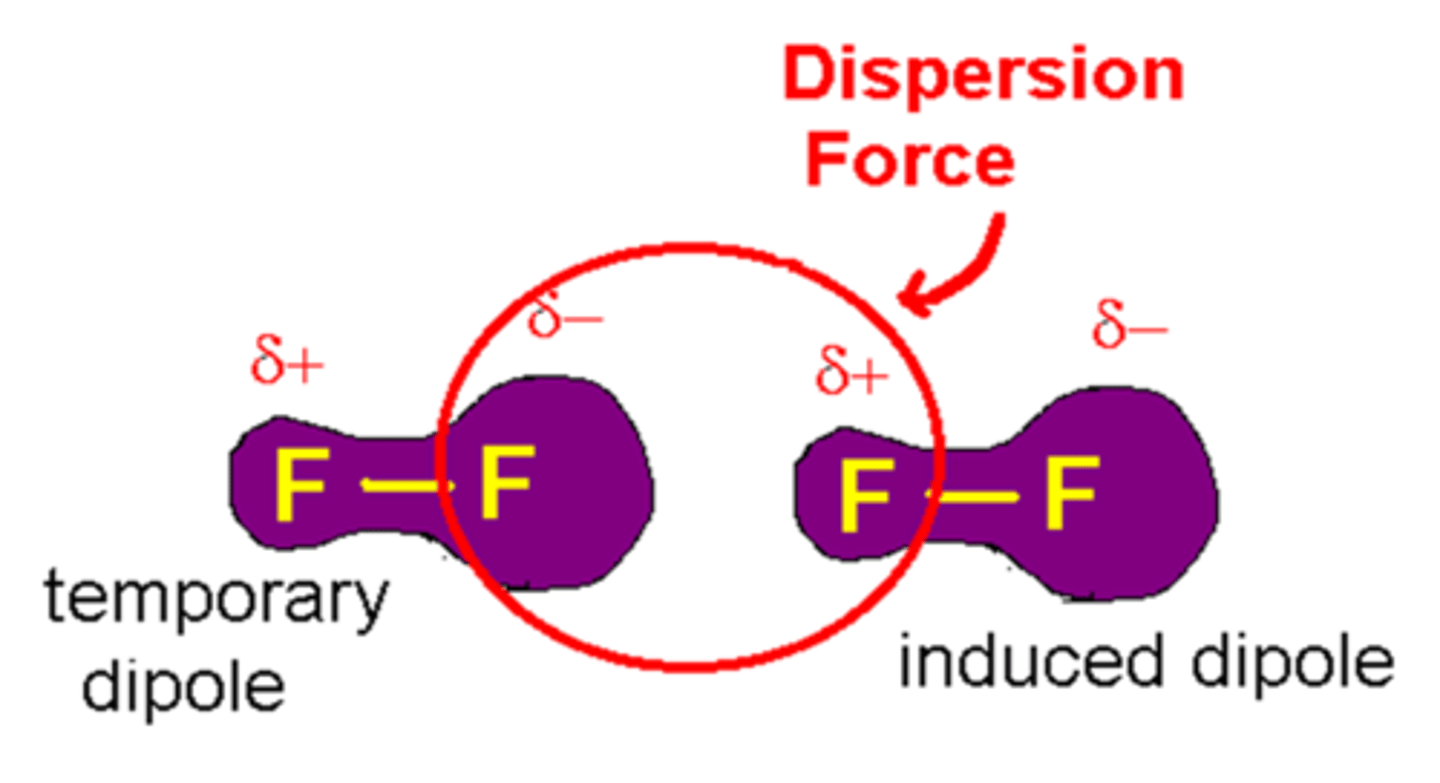
Dispersion Forces
The weakest intermolecular force. They intrinsically exist between all molecules (doesn't have to be ionic/polar) and occur due to the movement of electrons forming temporary dipoles. The more electrons a molecule has, the stronger the dispersion forces.
Physical Properties due to bonding and intermolecular forces
Polar covalent/Ionic compounds have strong dipole-dipole interactions between them, while Nonpolar compounds have only weak dispersion forces. The stronger a bond is, the more energy is required to break it, so ionic and polar compounds tend to have higher melting and boiling points than nonpolar compounds.
E.g. Water, a polar covalent compound, boils at 100°C while sodium chloride, an ionic compound, has a much higher boiling point at 1413°C.
Chemical structures
Ionic network, Covalent network, Covalent Molecular and Metallic.
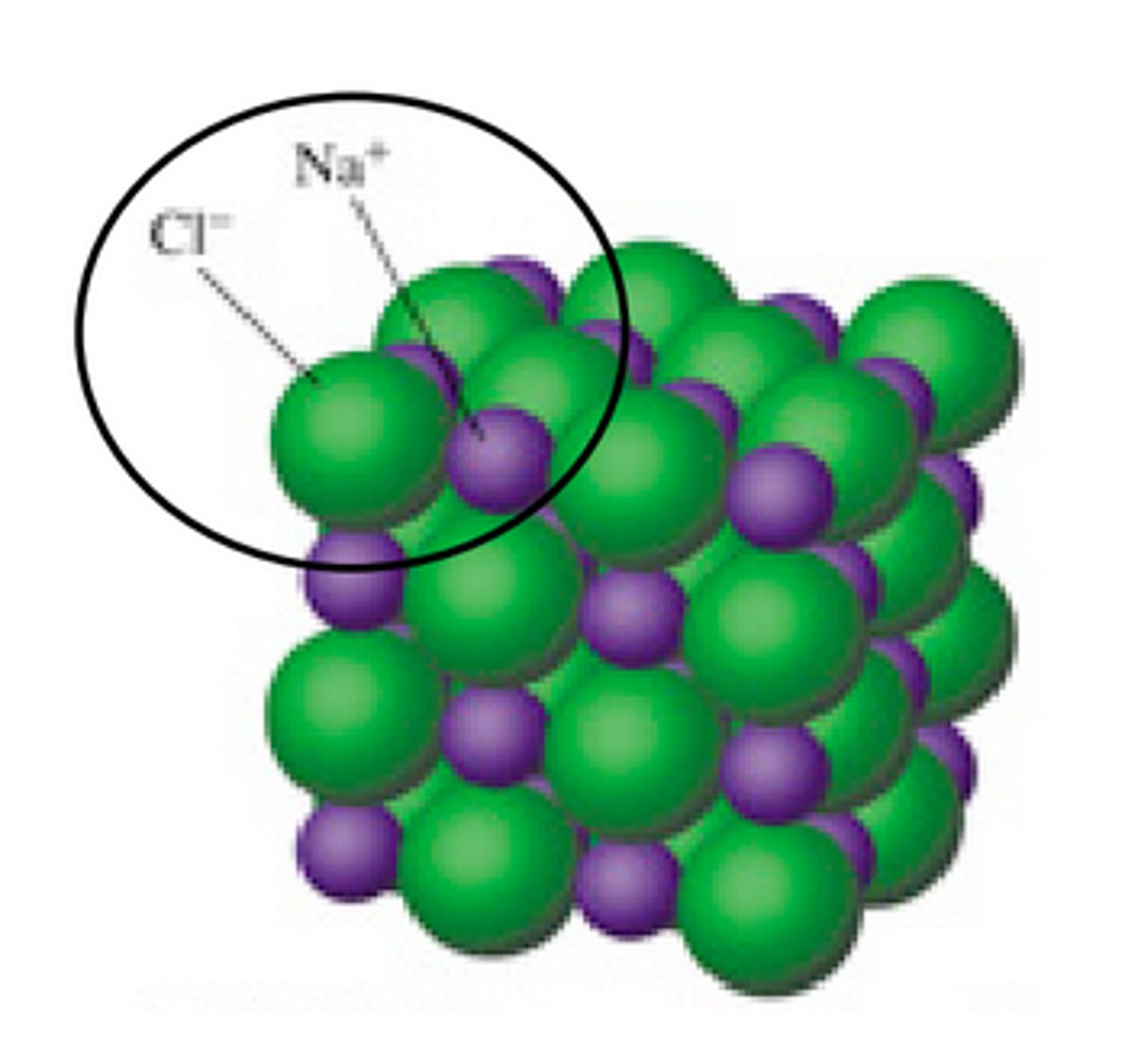
Ionic Network
Ionic compounds form lattice structures, where each cation is surrounded by 6 anions, and each anion is surrounded by 6 cations - forming something like a cube like NaCl does.
Ionic solids have poor electrical conductivity - they have no free electrons, because they are locked into the oppositely charged atoms, and the ions are stuck in a lattice structure.
Covalent molecular
Discrete molecules, with weak intermolecular forces between each molecule.
Covalent Molecular substances do not conduct electricity because there are no ions or free floating electrons.
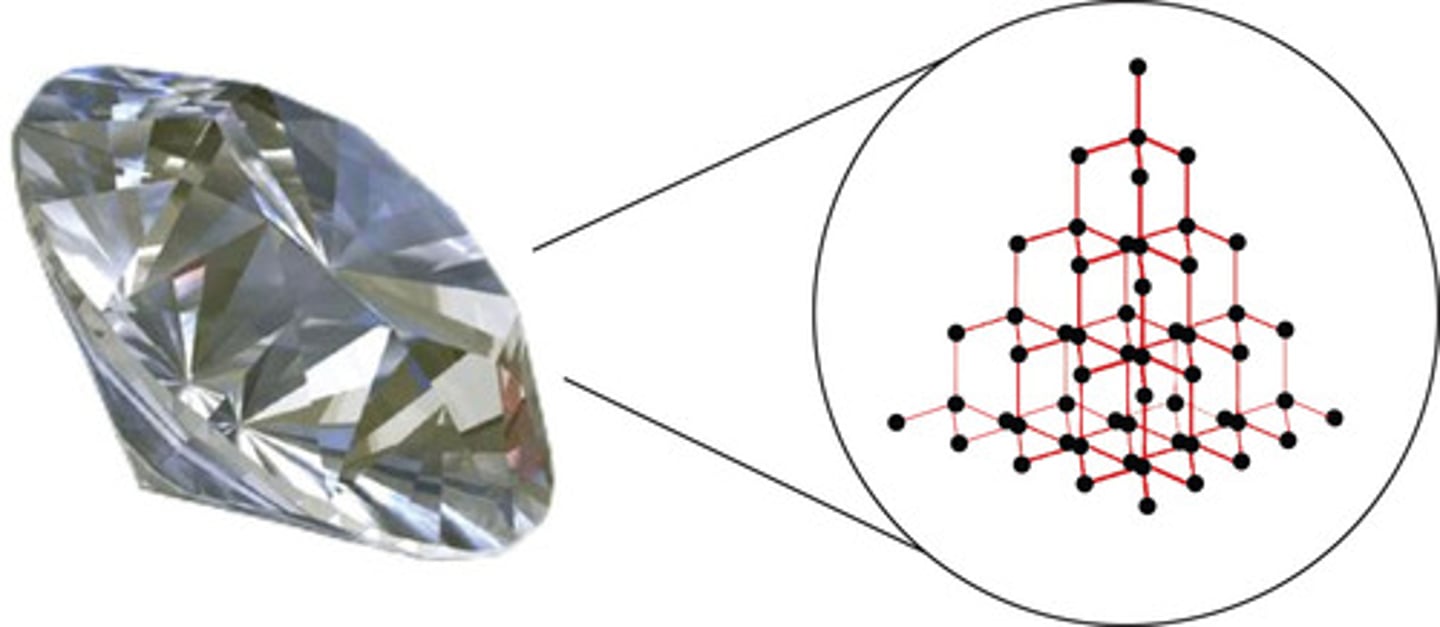
Covalent Network
Can be 2D and 3D - most prominent example is Graphite (2D Network) vs Diamond (3D Network). They are both different structures of the same element and have very different properties because of their different structures.
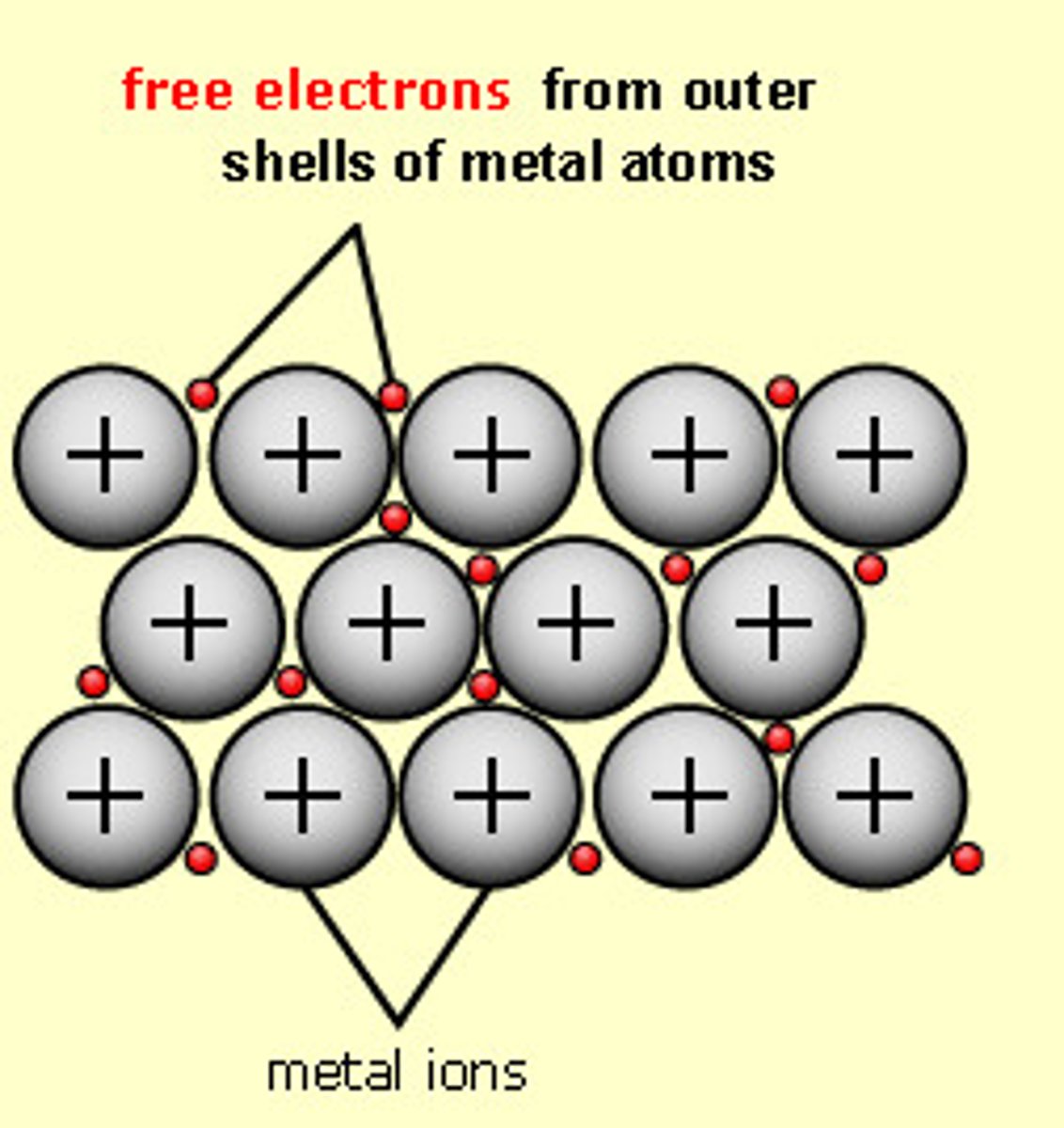
Metallic
A lattice of positive metal ions are held together by delocalised electrons (electron cloud/ electron sea) in metallic lattices.
The delocalised electrons carry electricity very well, making metals great conductors of electricity.
They also carry heat energy in the form of kinetic energy by moving around and hitting the colder parts of the lattice, making metals great conductors of heat
Delocalised electrons are also responsible for the malleability/ductility of metals
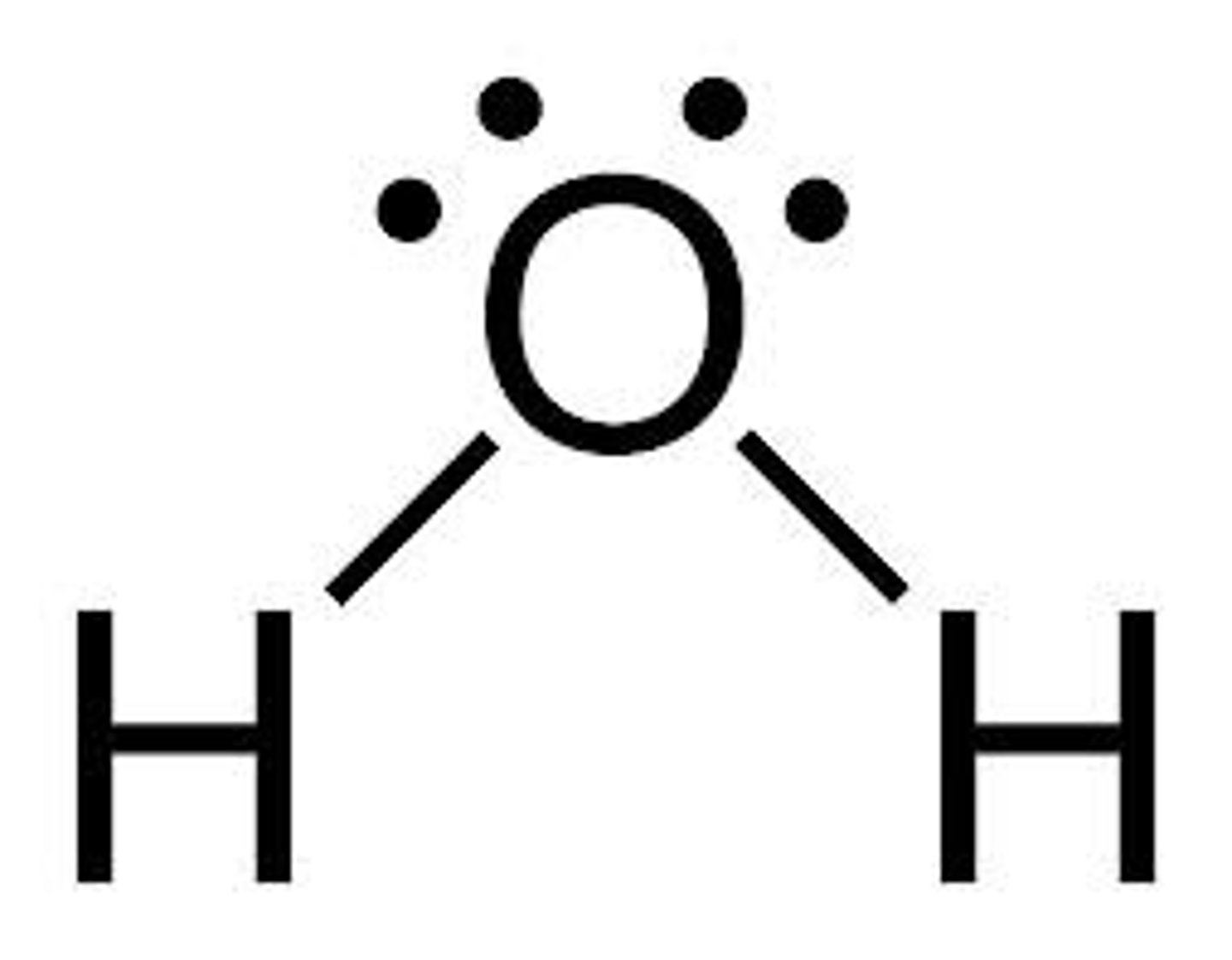
Valence shell electron pair repulsion theory - CO2 AND H2O
The shape of a molecule depends on the electron configurations of its atoms - theory.
E.g. CO2 has its nonbonding electrons distributed equally on opposite sides (it is nonpolar), so has a linear structure, since the repulsion is equal on either side.
H2O has it's nonbonding electrons on top of oxygen, so they repel the hydrogen atoms downwards. As the hydrogen atoms come closer together, they also repel each other, creating a bent molecular shape
Stoichiometry
Since the Law of Conservation of Mass states that matter cannot be created or destroyed, the atoms before and after the reaction must be the same.
Thus, Chemical Equations can be balanced by adding stoichiometric coefficients in front of the molecules.
E.g.2Na(s)+Cl2 (g)→2NaCl(s)
This means that for every two separate Sodium atoms (total mass: 45.98 amu) and diatomic Chlorine molecule (70.90 amu) that react, therefore ratio is 2:1.
Molar Mass Formula
n = m/MM
Number of Moles = Mass(g) ÷ Molar Mass(g/mol)
Concentration Formula
n = CV
(Number of Moles = Concentration of Solution (moles/litre) * Volume (litres))
Limiting reagents
Substance that is totally consumed when the chemical reaction is complete. The amount of product formed is limited by this reagent, since the reaction cannot continue without it, and will leave an excess of another substance.
Concentration
C1V1 = C2V2
*Convert concentrations to mol/L if required in calculations
Avogadro's Gas Law
When measured at the same temperature and pressure, equal volumes of different gases contain the same number of molecules.
V1/ n1 = V2/ n2
Charles' Law
The volume of a gas is directly proportional to its temperature when measured at a constant pressure. Convert celsius to kelvin.
V1/ T1 = V2/ T2
Gay-Lussac's Pressure and Temperature Law
The pressure exerted by a gas is directly proportional to its temperature (in Kelvin), when
Volume is constant. - Kevlin instead of celsius
P1/ T1 = P2/ T2
Boyle's Law
The pressure exerted by a gas is inversely proportional to its volume when measured at a constant temperature.
P1V1 = P2V2
Kelvin to celsius
celsius + 273.15 = kelvin
Combined Gas Law
P1V1/T1 = P2V2/T2
Temp always in Kelvin
STP = standard temp (273 K)
Standard Solutions
Known and fixed concentration.
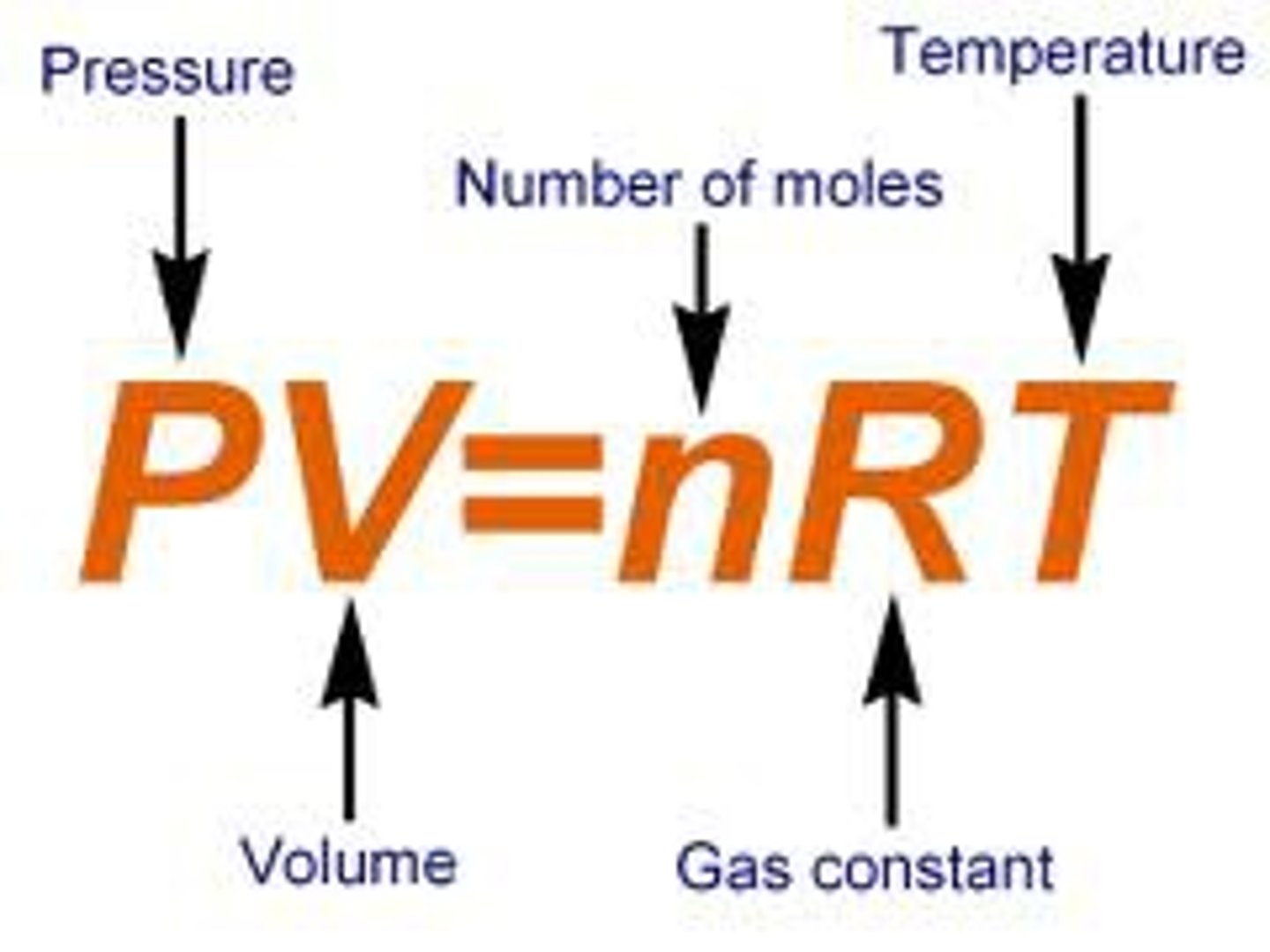
Ideal Gas Law
Theoretical model of a gas that obeys the gas laws exactly. It follows certain assumptions:
- gas molecules move randomly in straight lines
- pressure is due to collisions between the walls of the container and the molecules
- no intermolecular forces between gas molecules
- gas molecules themselves take up no volume
NOT ACTUALLY TRUE FOR GASES
PV = nRT
R is the universal gas constant: 0.082 K⋅mol/L⋅atm:
Electrolysis
Application of an electric current to decompose a compound Consider: 2H2O → 2H2 + O2
Requires much more energy than boiling water, since it's breaking covalent bonds instead of only the intermolecular bonds. It is also a chemical reaction as bonds are being broken and reformed, while boiling water is a physical change.
Chemical Reactions
When chemical bonds are broken, rearranged and established to form new substances. They involve reactants turning into products.
Indicators of a chemical reaction
1. Bubbles
2. Colour change
3. Change in Energy (change in temperature)
4. Appearance of a solid (due to precipitation)
5. Disappearance of a solid (not due to dissolving)
6. Formation of new substance ← this is the only thing that guarantees a chemical reaction occurring,
the rest are indicators
Exothermic reaction
The reactants have more energy than the products
Energy is released from bonds being formed and goes into the surroundings, usually causing the temperature to rise.
E.g. Combustion
Endothermic reaction
Energy is taken from the surroundings and the reactants have less energy than the products. Because energy is being used to break bonds, endothermic reactions usually cause the temperature to drop.
E.g. Decomposition
Synthesis Reaction
Formation of compounds by combining simpler substances such as elements.
Exothermic (energy is given off when bonds are broken).
A + B → AB
E.g. 2Na + Cl2 → 2NaCl
Displacement Reaction
When salts dissolve in water, the bonds between the cation and anion separate inside the solution.
This swapping of ions occurs when two salt solutions are mixed.
Reactants have to be soluble.
AB + CD → AD + CB
E.g. Na2S + 2HCl → 2NaCl + H2S
Precipitation Reaction
New insoluble salt is formed and falls to the bottom if heavy enough, new solid particles formed are called the precipitate.
Soluble Salt (aq) + Soluble Salt (aq) → Precipitate (s) + Soluble Salt (aq)
E.g. AgNO3 + NaCl → AgCl + NaNO3
Decomposition Reaction
When atoms of a compound are separated to form two or more products.
AB → A + B
E.g. NaCl→Na+Cl
Neutralisation Reaction
Acid + Base → Salt + Water
E.g. HCl + NaOH → NaCl + H20
Acid + Metal Reaction
Acid + Metal → Salt + Hydrogen gas
E.g. Mg + 2HCl → MgCl2 + H2
Metal Carbonate + Acid Reaction
Metal Carbonate + Acid → Salt + Carbon Dioxide + Water
E.g. 2HCl + Na2CO3 → CO2 + H2O + 2NaCl
Metal Oxide + Acid Reaction
Metal Oxide + Acid → Salt + Water
E.g. CuO+2HCl→CuCl2 + H2 O
Combustion Reaction
Hydrocarbon + Oxygen → Carbon Dioxide + Water
Incomplete forms → Carbon Monoxide + Carbon + Water
E.g. Complete Methane CH4 + 2O2 → CO2 + 2H2O
E.g. Incomplete for Ethane C2H6 + 2O2 →CO + C + 3H2O
Aboriginal Detoxifying Poisonous Foods
Heating: The cut-up cycad fruit or seeds are heated, which helps to decompose some of the toxic compounds, including macrozamin, reducing their toxicity before further processing.
Fermentation: The outer flesh of the cycad fruit, which contains macrozamin, undergoes anaerobic fermentation (soaking and burial), allowing microbial activity to break down macrozamin into less harmful substances. This process is especially important even if the fruit is ripe, as it ensures any remaining macrozamin is neutralized.
Leaching: The seeds, which contain cycasin, are cut open and soaked in running water for several days. Cycasin is water-soluble, so it dissolves and diffuses out of the seeds into the flowing water, which carries the toxin away and prevents it from re-entering the seed. Prolonged leaching is crucial to ensure thorough removal of cycasin.
Final Preparation: Once detoxified, the seeds are ground into a meal and baked to make flat bread, providing a safe and nutritious food source.
Reactivity Series of Metals
Pascal Siakam Cut My Ancient Zebra Into Large Chunks Making Super Glue.
K, Na, Ca, Mg, Al, Zn, Fe, Pb, Cu, Hg, Ag, Au
Metal Reactivity in terms of Periodic Trends
Since metals want to give away electrons, the more reactive metals give away electrons more easily.
Thus, metals with larger atomic radius (and therefore lower ionisation energies, and lower electronegativity) are more reactive.
Rate of Reaction
Measure of how quickly reactants are turned into products - it is represented by a change in the concentration of reacts/products with time.
Links to collision theory (must collide with correct orientation and energy)
Way to Increase RoR
Increasing Temperature: gives the particles kinetic energy for collision
Increasing Concentration: more particles of reactant in the same space, so collisions are more likely
Increase the Surface Area: more of one reactant is exposed to the other, so more collisions
Add a Catalyst: a substance which speeds up the reaction by decreasing the activation energy required
Increasing Pressure: which pushes particles closer together, as increasing pressure decreases volume
Activation Energy
Minimum amount of energy that is required for a set of reactants to begin reacting.
When met the existing bonds break and reactants collide at high enough speeds
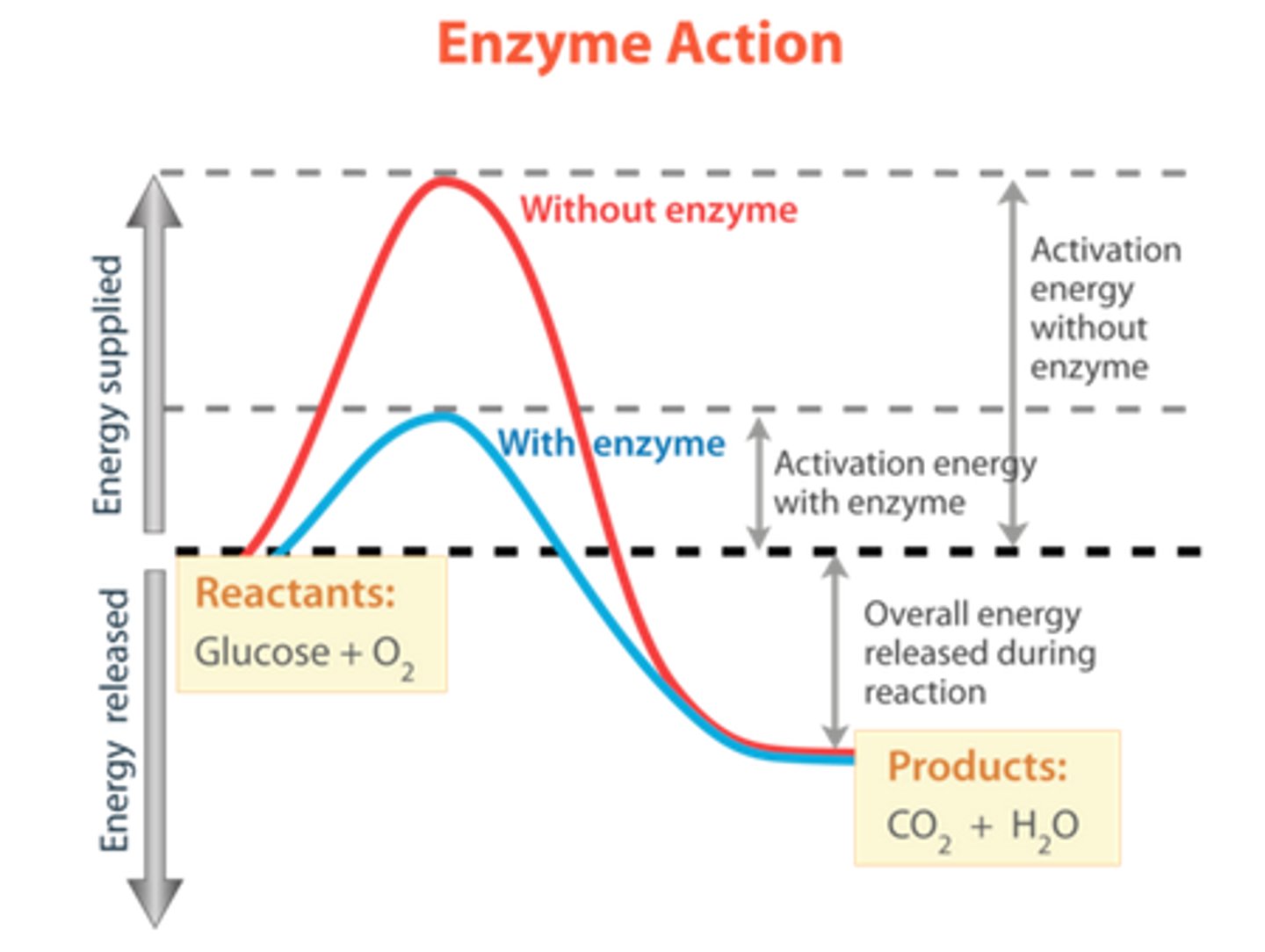
Activation Energy Curve
The activation energy is the peak of the curve. A catalyst (such as an enzyme) decreases the activation energy required - blue
Energy Changes in Chemical Reactions
All chemical reactions have some energy changes occurring, since bonds are being broken and formed.
● The formation of bonds produces energy
● The breaking of bonds consumes energy
These energy changes follow the law of conservation of energy - the things storing Chemical Potential Energy are:
● Chemical Bonds
● Intermolecular Forces
● Repulsion between Electrons
● Repulsion between Nuclei
● Kinetic Energy of particles
Thus, the energy of the system isn't the same before and after the reaction - some reactions release energy, while some consume energy (i.e. exothermic and endothermic reactions). This is dependent on what and how many bonds are being broken/formed.
Enthalpy
Enthalpy is defined as the energy content of a system.
It is very difficult to know the exact enthalpy of a substance, so the enthalpy change is measured/calculated instead.
Measured as J/mol.
ΔH = -Q/n
+ve enthalpy = endothermic
-ve enthalpy = exothermic
Hess' Law
Hess's Law states that the total enthalpy change for a chemical reaction does not depend on
the pathway that it takes - it only depends on the initial and final states.
The total enthalpy change for a reaction carried out in multiple steps is also equal to the sum of the enthalpy changes of each individual step.
Energy Profile Diagram
Energy Profile Diagrams are representations of the energy (or enthalpy) level of the reaction as the reaction progresses.
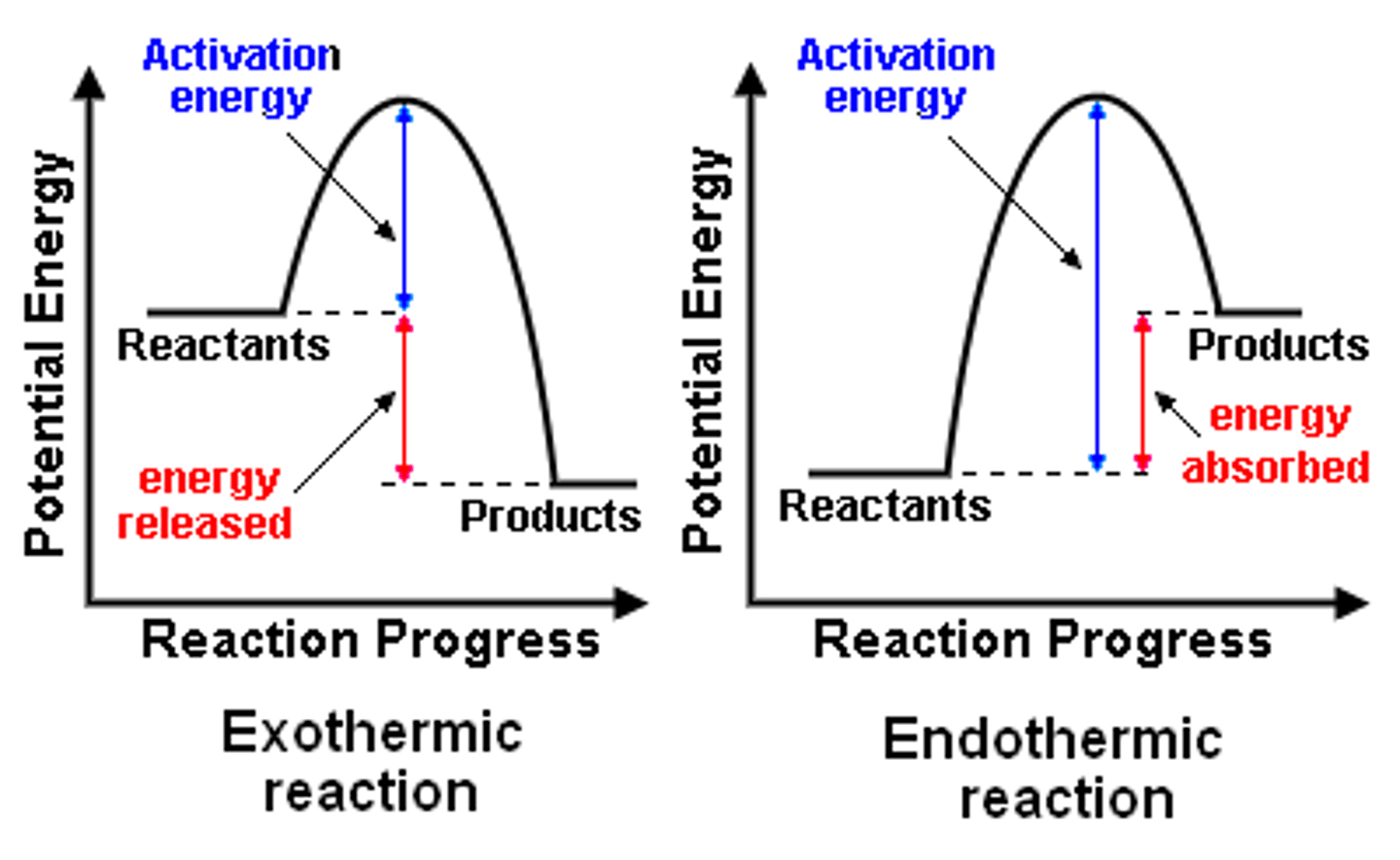
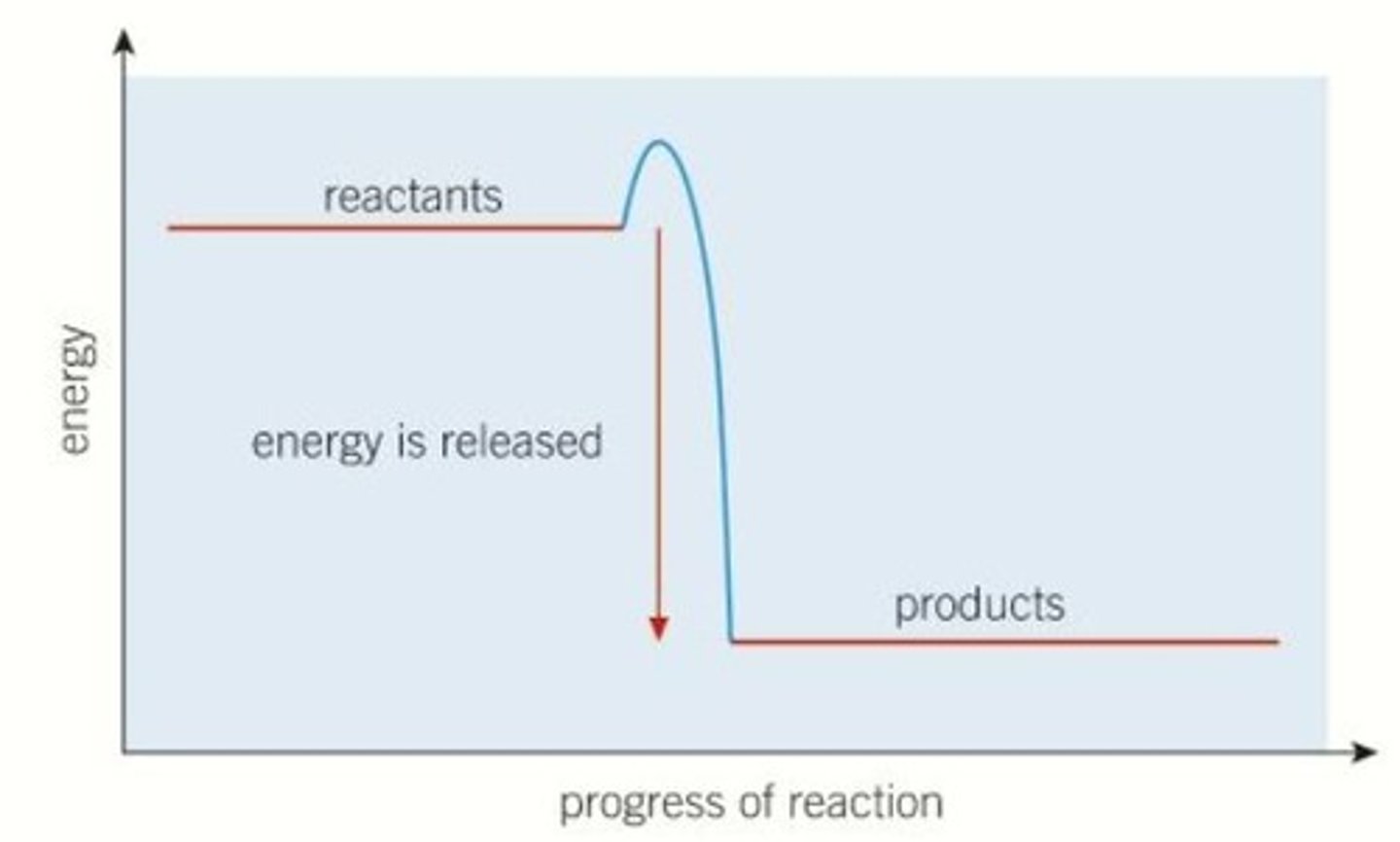
For example, it might start at one point, then gain energy as bonds are broken, then release energy as new bonds are formed, until the final products have an net lower enthalpy than the initial reactants. This shows that the reaction has overall released some energy, making it exothermic
If the Energy of Products < Energy of Reactants, it is exothermic (because that energy is released as heat), and ΔH < O.
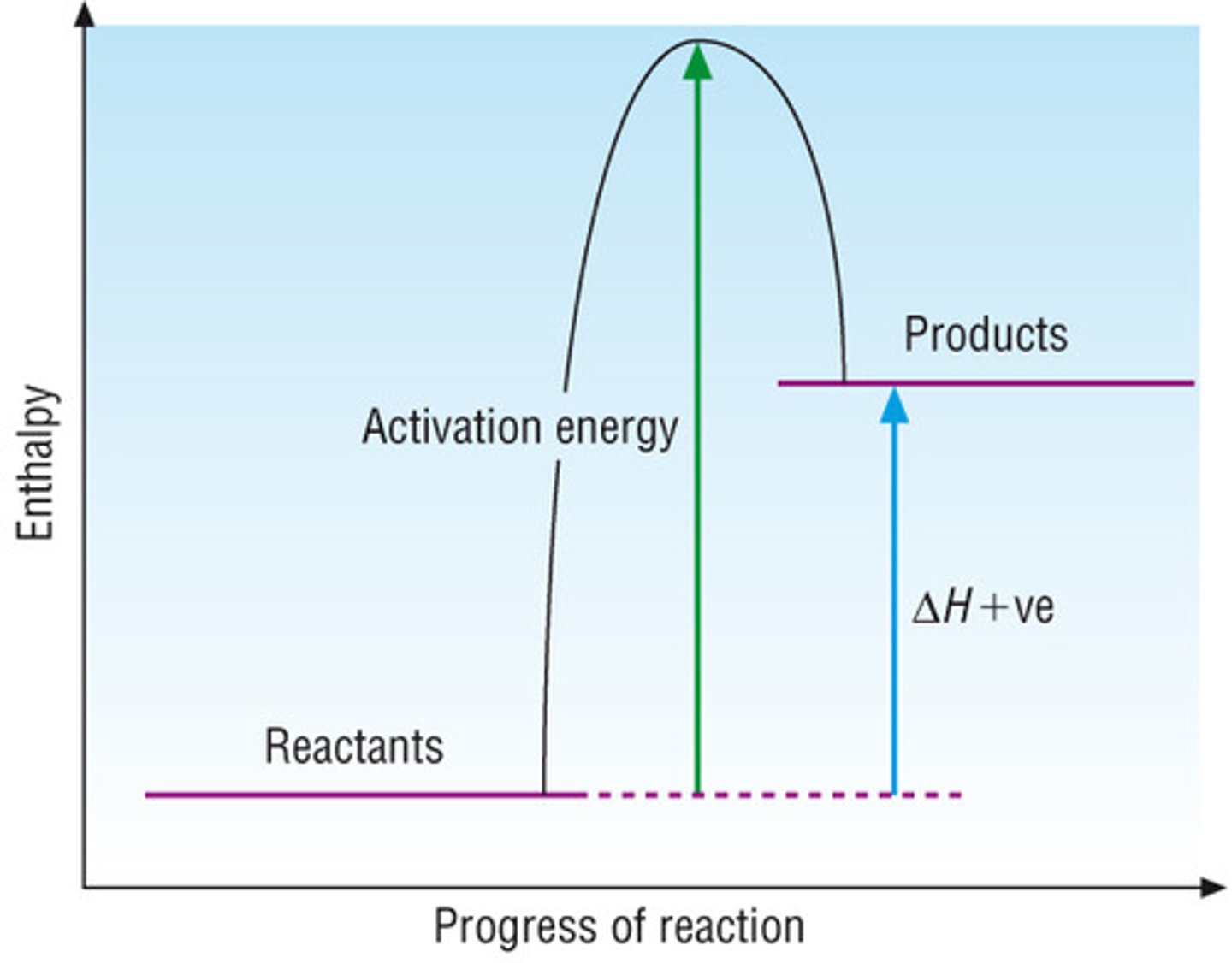
If the Energy of Products > Energy of Reactants, it is endothermic (because additional energy is consumed), and ΔH > O.
Also note that the Activation Energy in an exothermic reaction is generally lower than an endothermic reaction, because endothermic reactions are actually consuming energy.
Bond Energy
Bond Energy/Enthalpy refers to the amount of energy required to break the bonds of 1 mol
of a substance into its constituent atoms under STP.
E.g. Given that an N-N triple bond has a bond energy of 945 kj/mol, H-H bond has a bond energy of 436 kj/mol, and N-H bond has a bond energy of 391 kj/mol, calculate the enthalpy change of the following reaction:
N2 + 3H2 → 2NH3
Energy consumed to break bonds = 1 x 945 + 3 x 436 = 2253
Energy released by new bonds forming = 6 x 391 = 2346
ΔH = +2253-2346 = -93 kj/mol (exothermic)
Therefore,
ΔH = ∑ ΔH(bonds broken) - ∑ ΔH(bonds formed)
Standard Enthalpy of Formation
The standard enthalpy of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements, with all substances in their standard states.
Given the standard enthalpies of formation(ΔHf) of different substances, the change in enthalpy of a reaction can be calculated:
ΔH(Reaction) = ∑ΔH(Products)− ∑ΔH(Reactants). Remember to multiply by the coefficients.
Stepped Reactions
A stepped reaction is one that occurs in multiple steps. When given information about the enthalpy changes for each step, the total enthalpy change of the reaction can be calculated.
E.g. Calculate ΔH for the following reaction: 2CO + O2 → 2CO2 2C + O2 → 2CO, ΔH = -221.0 kJ/mol
C + O2 → CO2, ΔH = -393.5 kJ/mol
1. Manipulate the given reactions to resemble the actual reaction
a. 2CO → 2C + O2 (equation flipped so flip ΔH, ΔH = 221.0 kJ/mol)
b. 2C + 2O2 → 2CO2 (equation x2 so ΔH x2 as well, ΔH = -787 kJ/mol)
2. Add the manipulated reactions
a. 2CO+2C+2O2 →2C+O2 +2CO2
b. ΔH is added too, so 221 - 787 = -566 kJ/mol = ΔH of this reaction
3. Cancel out the same compounds on both sides, then check if the equation matches the original
a. Cancel out 2C and one O2
b. 2CO + O2 → 2CO2 is left, this matches the initial reaction
c. So the answer is ΔH = -566 kJ/mol
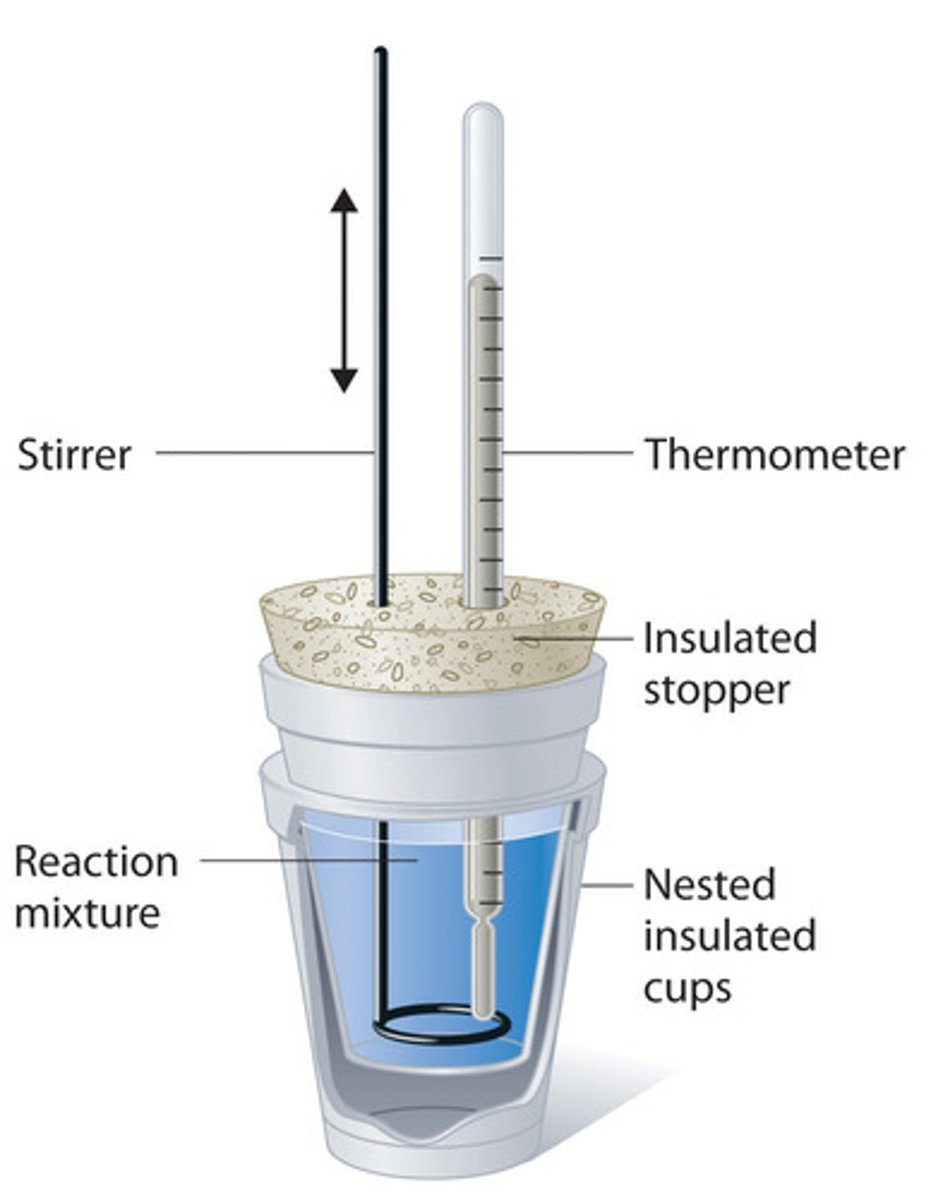
Calorimetry
A calorimeter is a device used to measure the quantity of heat flow in a chemical reaction. The type shown in the diagram below is a "coffee cup calorimeter", used to measure neutralisation and similar reactions
● It consists of a covered container surrounded by insulation (such as styrofoam), to minimise heat getting in from the surroundings, because it only wants to measure the heat flow in the chemical reaction.
● A thermometer measures the temperature of the system as the reactants are stirred.
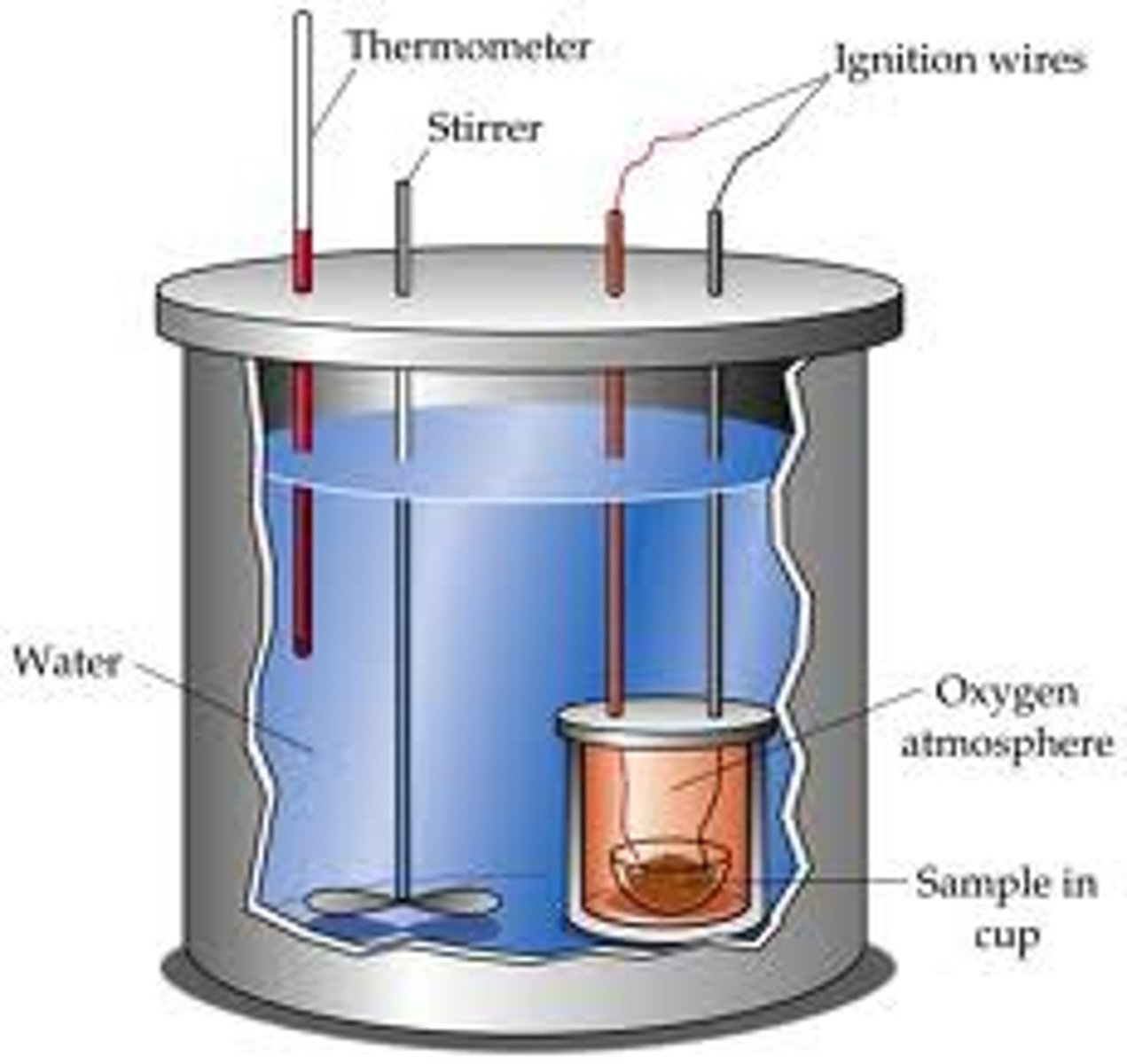
Bomb calorimeters
are a more effective alternative to accurately determine enthalpy changes in combustion reactions. This encompasses a copper 'bomb' containing measured amounts of reagents, which is submerged in a calorimeter of water. The chemical is ignited,
and the heat released warms the water, making use of the specific heat of water to determine change in energy within the system.
specific heat formula
can be used to calculate the heat/enthalpy change:
Q = mc∆T
Q = heat energy (Joules, J)
m = mass of a substance (g - this is can also be kg depending on the units used in specific heat capacity)
c = specific heat capacity (units J/g/K)
∆T = change in temperature (Kelvins, K, or Celsius, C - this would be the same)
The specific heat capacity of a substance is the amount of energy needed to increase 1 gram of that substance by 10 C.
● Note in bomb calorimeters the mass and specific heat capacity of water should be used as m and c
Molar Heat of Combustion
refers to the energy released when 1 mole of a substance undergoes complete combustion under STP - it is measured in kJ/mol. This is always a positive value, as combustion is an exothermic r eaction, and energy is released.
● The Molar Enthalpy of Combustion is always a negative value, because it is the change in energy of the system per mole of substance that undergoes combustion.
One method used to determine enthalpy of combustion of liquid fuels (eg. ethanol, methanol) is to burn the fuel in a spirit burner, and using the heat from the combustion of the ethanol to heat a measured volume of water, held in a double-walled copper calorimeter.
● Copper ensures efficient heat transfer, as it is a conductor, but it is often double-walled to prevent the heat exiting the calorimeter
● Make sure the flame is touching the calorimeter
● Leave only a hole at the top for the thermometer
Calculating Molar Heat of Combustion
The total amount of heat released by a substance is the number of moles of that substance x the molar heat of combustion:
Q = mcΔT
Q = nHC (when there is 100% heat transfer)
Enthalpy of Combustion:
use ∆H = -mc∆T/n NOTE: m = mass of water used, not of the fuel
Unfortunately, the results of this technique are not very accurate; a great deal of heat can be lost in the surroundings, and the combustion may be incomplete (indicated by the deposition of black soot on the bottom of the water container).
Exothermic energy profile diagram
Endothermic energy profile diagram
Validity
1. Controlled variables
2. Does it answer the aim?
3. Not repeated unreliable therefore invalid is it accurate
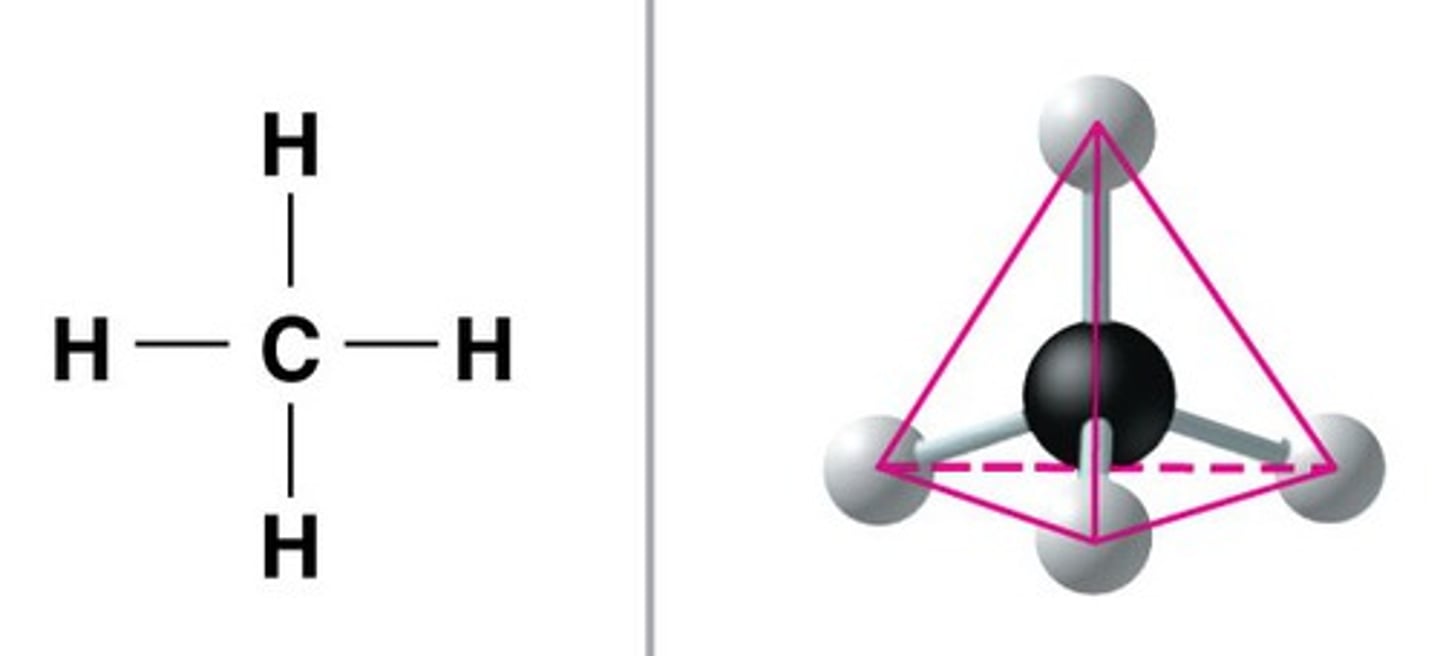
Covalent molecular
Covalently bonded molecules held together by weak intermolecular forces
Why does calorimeter experiment not produce results close to the published values?
As heat is lost to the surrounding air, to the container holding water. Sometimes combustion is incomplete.
Percentage Error formula
energy absorbed during the experiment / actual value (secondary source) x 100
Enthalpy Change

Nonmetals
Nonmetals are (usually) good insulators of heat and electricity, are brittle; usually dull many of the elemental nonmetals are gases at room temperature, while others are liquids and others are solids.
Metalloids
Metalloids have properties of both metals and nonmetals, and can be made to conduct electricity in some circumstances.