p1.2 - changes of state
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
what does temperature tell you
the average kinetic energy of the particles
what does the energy in a thermal store depend on
the arrangement of particles and how fast they are moving / vibrating
what 3 things can heating do
change the energy stored within the system to increase temperature
produce a change of state
make chemical reactions happen
what is a physical change
when no new products are made and the particles are just rearranged
they are easily reversible
what is a chemical change
when atoms are joined in a different way
it is not easily reversible
what 3 things does amount of energy needed to raise the temperature of an object depend on
the material of the object
the temperature change
the mass of the material
what is a specific heat capacity
the energy required to raise the temperature of 1kg of a material by 1 degrees celsius
what are the 6 changes of state
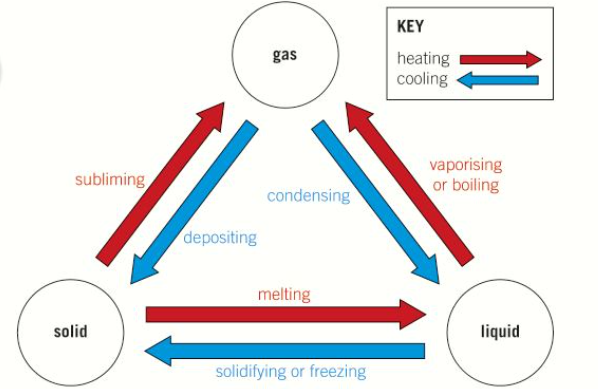
why does temperature not increase when a substance is changing state
the internal energy is still increasing, however its used for breaking bonds between particles to change state rather than to increase temperature
what is specific latent heat of fusion / melting
the energy required to change 1kg of a substance between the solid or liquid state
what is specific latent heat of vaporisation
the energy required to change 1kg of a substance from the liquid to gas state