2. Traditional preservation methods (DONE)
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
30 Terms
Three traditional ways of food stabilization
Reduction of water activity, antimicrobial composition
Thermal preservation
‘Non-thermal’ preservation
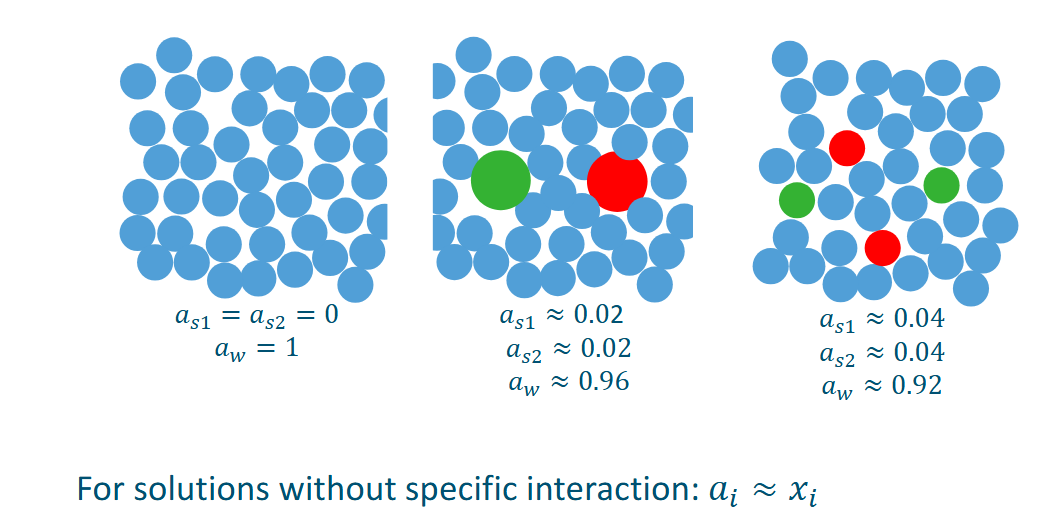
ai vs xi in solutions without specific interaction
Effect of solute on chemical potential
There are two chemical potentials one for the solute and one for the water
a is the activity of the water/solute
The chemical potential of a solute in a solution increases with higher solute concentration.
The presence of a solute lowers the chemical potential of the solvent
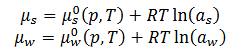
Differences in chemical potential
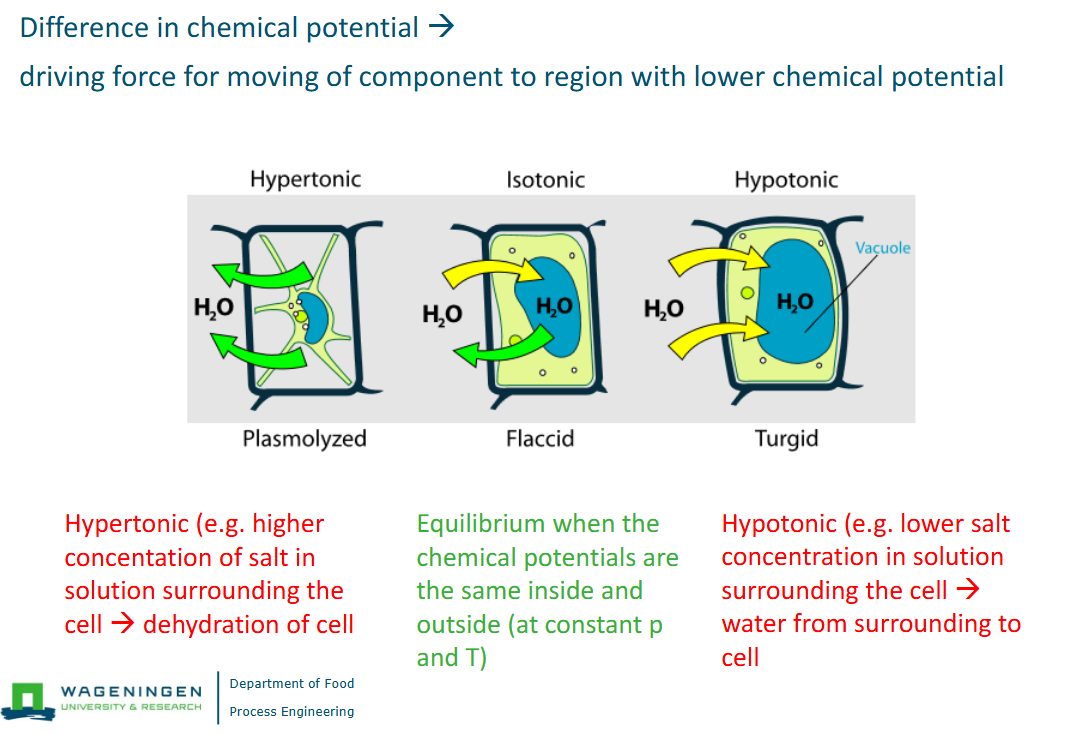
How to calculate the water activity of a solution
Calculate mole fraction of solute.
Calculate mole fraction of water
Calculate mole fraction with water on top and the rest on bottom.
Is addition of salt to a product effective for preservation?
A lot of salt has to be added to actually prevent the growth of microorganisms
This usually also changes the taste and therefore is not worth it.
Is sugar or salt more effective in reducing water activity?
Sucrose is less effective than NaCl
Additionally measured and calculated aw deviate for sucrose: measured < calculated.
What does it mean when 𝛾𝑤 is smaller than 1?
Meaning it is not an ideal solution, as there is an interaction between solute and solvent.
In sugar this happens due to strong interaction between the sugar OH groups and water → there is less ‘free water’
Which is good because it prevents microbial growth.
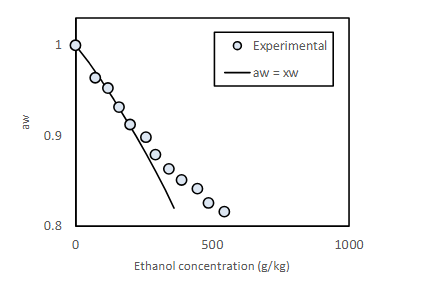
Effect of fermentation on water activity
Ethanol is produced, which lowers the water activity significantly
Sorption isotherm
Equilibrium between air and product
Creates controlled relative humidity
RH/100=aw
Measure change in mass
Temperature dependent
Axis for sorption isotherms
Moisture content (gH2O/100g DS) vs Water activity
Why does acidification prevent growth of microbes
Most microbes do not grow below 4.5
What acid is usually used for acidification?
Acetic acid
Cheap
Strong acidification
Also antioxidant (will reduce browning and oxidation of oils)
Other options:
Citric acid
Fumaric, lactic and malic acid
Phosphoric, tartaric acid
Preservative action of weak acids
Charged, dissociated ions can not penetrate the hydrophobic cell membrane
Un-dissociated acids (a-polar) can penetrate cell membrane passively
In cell, acids dissociate and establish lower intracellular pH
Cell tries to re-establish pH by actively expelling ions
Energy sources are depleted, cell gets exchausted and may die
Positive redox potential and spoilage
Usually spoiled by aerobic bacteria and fungi
How can spoilage be prevented from a redox perspective?
Creating a reducing atmosphere (anoxic, using nitrogen or CO2)
Or by adding reductors
Typical reductors: ascorbic acid, ascorbate, nitrite and sulphur dioxide.
Examples reductors
Ascorbic acid
Can reduce quinone into catechol and therefore stops enzymatic browning
Is antimicrobial even to facultatively anaerobic organisms.
Sulphur dioxide
Used in wines to stop growth of yeast
Used in dried fruits
Nitrites
Active against anaerobic bacteria
Can create ions that inhibit bacterial growth
Caustic
Also known as lye (hydroxides of Na, K or Ca)
has a high pH which will saponify lipids thus rendering bacterial membranes defective, and also partially hydrolyze proteins.
Preservatives against gram + microorganisms
Lysozyme
Nisin
Four types of smoking used as a preservation method
Cold smoking
Hot smoking
Roast smoking
Application of liquid smoke
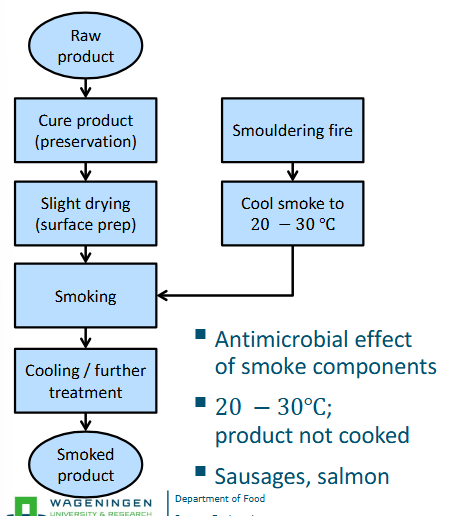
Steps of cold smoking
Steps of hot smoking
Curing = drying by means of salt, nitrates, sugars, etc.
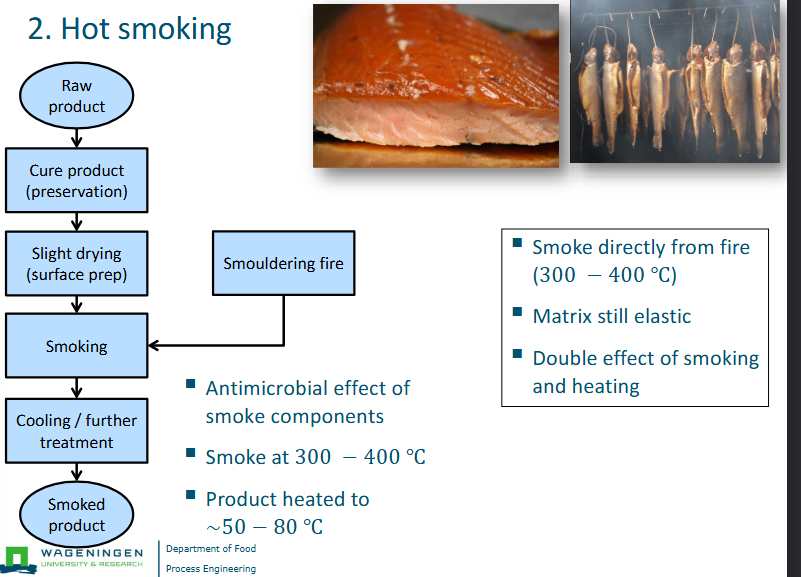
Roast smoking
Barbeque
Product roasted
In some cases: product desiccated (e.g. Bakkwa)
Can be stored without refrigeration
Smoking with smoke condensate
Normal smoke contains toxic components
Smoking takes time: expensive
Alternative:
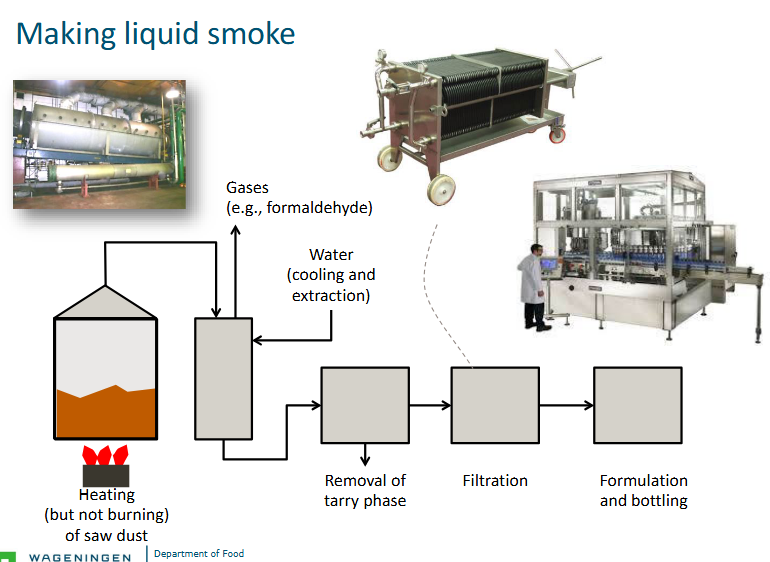
Smoke condensate or ‘liquid smoke’: condensed/dissolved and refined
Controlled pyrolysis of sawdust
Vapor led through cold tube with water (‘stripping’) - components condense and dissolve/disperse in the water
Some volatiles stripped away
Phase speparation (purification); removes tarry phase (such as PAHs), particles settle
Aqueous phase may be concentrated.
Making liquid smoke
Tarry: The tarry phase in smoke is the solid and liquid particulate matter that results from the combustion of substances like tobacco or biomass. This is a toxic residue containing a variety of harmful chemicals, including carcinogens, and is a major cause of smoking-related diseases by coating the lungs and damaging them over time.
Hurdle technology
Using several mild preservation strategies at the same time
Disruption of homeostasis
Conditions allow metabolic activity, but are not ideal (pH, T, E)
Metabolic exhaustion
Spore germination is possible, growth is hindered by metabolic exhaustion
m.o. live but do not grow
Several simultaneous stress factors avoid build-up of tolerance by micro-organisms.
Compartmentalization as preservation method
Water-in-oil emulsions
Smaller droplets
Better microbiological stability, local/isolated growth only
More difficult to physically stabilize
Physical barrier against outgrowth of microorganisms
Butter, margarine, oil-continuous sauces, are stable, do not need refrigeration.
How does smoke preserve foods?
Contains antimicrobial component (e.g. phenolics, formaldehyde & acetic acid)
Acetic acid also provides lower pH
High temperatures
Example of hurdle technology
Curing of meat is a traditional form of hurdle technology, combining drying, salting, pH reduction (fermentation), and nitrites to inhibit C. botulinum and enhance color
Antimicrobials spices
Herbs and spices such as thyme and rosemary contain potent antimicrobials.