Chapter 5: Thermal Effects
Moving Particles
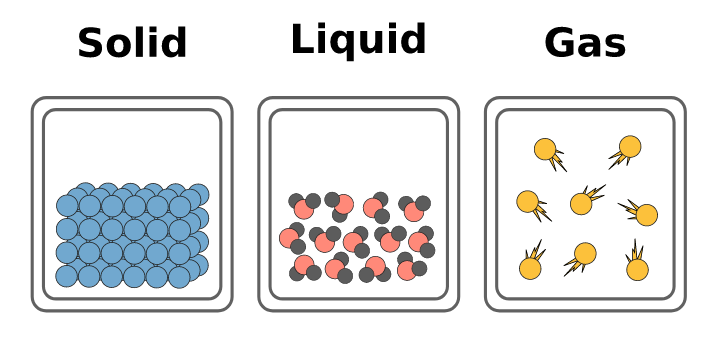
Solids, Liquids and Gases
{{Solids:{{
- Have a fixed shape and volume.
- Particles are held tightly together by strong forces of attraction.
- They can vibrate back and forth, but cannot change shape.
{{Liquids:{{
- Have a fixed volume but no fixed shape.
- Particles are close together and attract each other.
- They vibrate so vigorously that the attractions cannot hold them in fixed positions and can move past each other.
{{Gases:{{
- Have no fixed shape or volume, and can quickly fill any space.
- Particles are very well spaced out, and free of any attractions.
- They move about at a high speed, colliding with each other and the walls of their container.
Kinetic Theory
==It states that matter is made up of tiny particles which are constantly in motion. The particles attract each other strongly when close but the attractions weaken if they move further apart.==
Brownian Motion
- ==The kinetic theory explains Brownian motion as follows. The bits of smoke are just big enough to be seen, but have so little mass that they are jostled about as thousands of particles (gas molecules) in the surrounding air bump into them at random.==
Temperature
The Celsius Scale
- Also known as centigrade scale.
- The numbers on the scale were specially chosen so that pure ice melts at 0`C and pure water boils at 100`C (under standard atmospheric pressure of 101 325 pascals).
- These are its two fixed points. Temperatures below 0 °C have negative (-) values.
Thermometers
==Temperature is measured using thermometers.==
One common type is of glass bulb thermometer; it contains mercury or alcohol.
- This thermometer is commonly known as clinical thermometer.
- Their range is 35 to 42 degrees Celsius. (89.6°F to 107.6°F)
Other thermometers for higher range of temperature readings we use:

Fixing a Temperature Scale
On the Celsius scale - 0 degrees Celsius (0 °C) is defined as the melting point of pure ice. This is the lower fixed point, known as the ice point, 100 degrees Celsius (100 °C) is defined as the boiling point of pure water, where the water is boiling under standard atmospheric pressure. This is the upper fixed point, known as the steam point.
NOTE: Both ice and water should be pure for accurate reading.
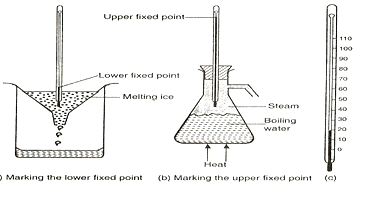
Liquid in glass thermometer
All liquids in thermometer expand
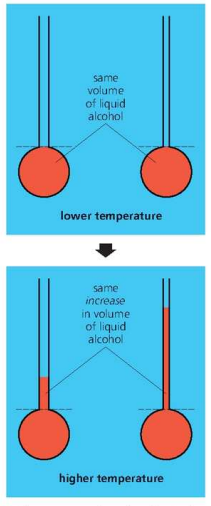
%%Sensitivity -%% %%narrower the tube, the higher the sensitivity of the thermometer.%%
- diagram below shows how width of bore affects sensitivity
%%Range -%% %%is the difference between maximum and minimum temperatures.%%
- To increase the range of a thermometer, use a wider bore or a longer stem.
%%Responsiveness -%% %%a thermometer with a larger bulb, or thicker glass round the bulb, is less responsive because it takes longer for the alcohol or mercury to reach the temperature of the surroundings.%%
%%Linearity%% %%- is when a given change in temperature causes the same change in length.%%
Heating Gases
How pressure changes with temperature (at constant volume)
==As the temperature of air rises, so does the pressure. This is because the molecules move faster.==
- There is a greater change in momentum when they hit.

How volume changes with temperature (at constant pressure)
==As the temperature rises, the volume of gas increases the gas expands.==

Thermal Conduction
==Conduction is the transfer of thermal energy.==
- More thermal energy is transferred if:
- the temperature difference across the ends of the bar is increased,
- the cross-sectional area is increased,
- the length of the bar is reduced.
- {{Thermal conductors:{{
- metals,
- silicon,
- graphite,
- etc.
- {{Thermal insulators:{{
- glass,
- water,
- wood,
- rubber,
- etc.
]]How materials conduct?]]
In atoms, there are tiny particles called electrons. Most are firmly attached, but in metals, some are loose' and free to drift between the atoms. When a metal is heated, these free electrons speed up. As they move randomly within the metal, they collide with atoms and make them vibrate faster. In this way, thermal energy is rapidly transferred to all parts.
Convection
==Convection occurs when molecules in a fluid with high thermal energy move to an area with low thermal energy.==
Convection in a Liquid
Occurs due to convection currents.
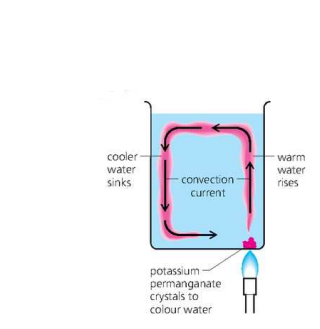
- As the water above the flame becomes warmer, it expands and becomes less dense. It rises upwards as cooler, denser water sinks and displaces it (pushes it out of the way). The result is a circulating stream, called a ^^convection current.^^
Convection in Air
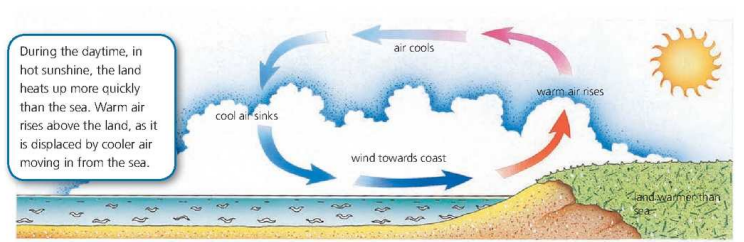
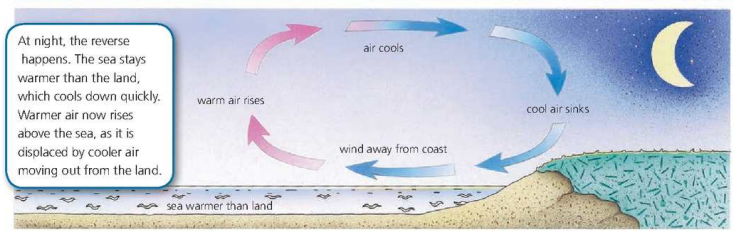
Thermal Radiation
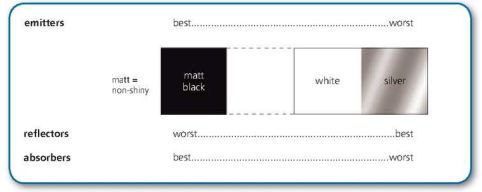
==Thermal radiation is electromagnetic radiation generated when heat from the movement of charges in the material is converted to electromagnetic radiation.==
White or silvery surfaces are poor absorbers because they reflect most of the thermal radiation away.
Liquids and Vapors
Evaporation
When a liquid ^^below its boiling point^^ changes into a gas.
- Factors affecting evaporation
- Higher the temperature faster the rate of evaporation.
- Greater the surface area faster the rate of evaporation.
- Increase in humidity slower the rate of evaporation.
- Higher the wind faster the rate of evaporation.
Boiling
==Boiling point is the temperature at which a given liquid will start vibrating and start to turn into a gas when heated.==
Condensation
==When a gas changes back into a liquid==
Specific Heat Capacity
==It is the amount of energy required to raise the temperature of 1kg of a substance by 1℃.==
Equation:

Latent Heat Capacity
==It is the amount of energy needed to change the state of 1kg of a substance.==
Equation:
