EOY 10 Topics 1,2,4,5,9
1/269
Earn XP
Description and Tags
atomic structure, bonding,structure + properties of matter, chemical changes, energy changes, atmospere
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
270 Terms
reactivity
how easily atoms lose,gain, or share electrons
how are ions formed
by loosing or gaining electrons or protons,they can be positive or negative
do atoms in group 4 form ions
no
do atoms with 1-3 electrons in their outer shell loose or gain electrons
loose electrons to form stable positive ions
do atoms with 5-7 electrons in their outer shell loose or gain electrons
gain electrons to form stable negative ions
do noble gas atoms react
no they are unreactive as they have full outer shells
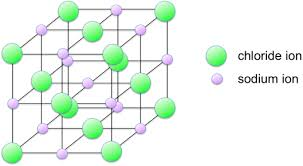
describe a giont ionic structure
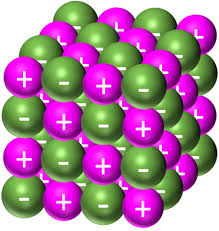
-giant ionic lattices
-regular structure
-ions attract each other (electrostatic attractions)in every direction with the lattice
-very strong
describe a simple molecular structure
-most covalent bonds have a simple structure
-strong covalent bonds between atoms
-week forces between molecules(intermolecular forces)
lustrous
sparkles in the light
lubricant
helps moving parts slide past each other
electrode
used to conduct electricity into a solution during electrolysis
why would a 1a metal be good conductors of electricity and heat (2 marks)
-pure metals have delocalised electrons
-which are free to move through the structure carrying heat or charge
what do delocalised electrons carry
heat or charge
why are 1b metals malleable (2marks)
-pure metals have regular layers of identical atoms
-these can slide over each other
what makes a metal malleable
regular layers of identical atoms- they can slide over each other
why are alloys harder than pure metals (3 marks)
-alloys are mixed mixtures ←
-different size atoms disrupts the regular arrangement
-layers can no longer slide over one another easily
why would something have high melting/boiling points/solid at room temp (2 marks)
-strong electrostatic attraction between positive metal ions and negative delocalised electrons
-which needs a lot of energy to break
why do ionic compounds conduct electricity as a liquid but not a solid (3 marks)
-ionic compounds have charged ions
-in solids they are in fixed position
-in liquids or in solution they are free to move and carry the charge through the structure
through shows that there is…
movement
compared to throughOUT shows NO movement
why is CO2 a gas at room temperature
-weak intermolecular forces so they don’t need lots of energy
-little thermal energy is needed
why don’t simple molecular structures conduct electrisity
-substances can only conduct electricity if they have charged particles that can move
-molecules are neutral and have no free electrons
-so no currant can flow
why do giant covalent structures have high melting points
-they have many strong covalent bonds
-require a lot of energy to overcome
why is graphite slippery
-weak forces between layers
-slide easily over each other
why is diamond hard
-giant structure
-4 strong covalent bonds per carbon atom
why is graphite softer than diamond (4 marks)
-diamond has a giant structure
-diamond has 4 strong covalent bonds per atom
-graphite has weak forces between layers
-weak intermolecular forces/weak forces
describe silicon dioxide (5)
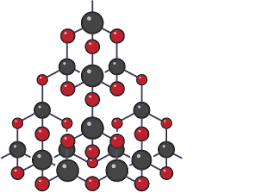
3d lattice structure (giant)
each silicon atom forms 4 covalent bonds with oxygen
-each oxygen atom forms 2 covalent bonds with silicon
-strong covalent bonds
-no delocalised electrons (all used in bonding)
describe diamond (3)
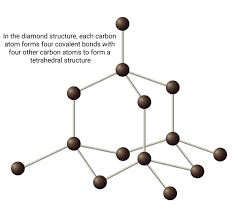
3d lattice structure (giant)
-each carbon forms 4 covalent bonds with other carbon atoms
-no delocalised electrons (all used in bonding)
describe graphite (5)
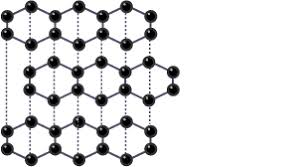
-same formular as diamond
-2d layers of hexagonal rings
-each carbon forms 3 covalent bonds with other carbon atoms
-1 delocalised electron on each carbon atom free to move through the structure
-weak forces between layers
properties of nanoparticles (3)
-large surface area to volume ratio
-high % of their atoms are exposed at the surface
-nano particles are highly reactive
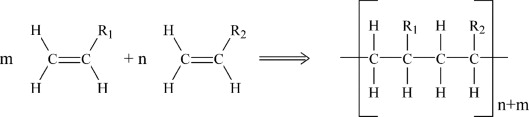
polymer
a long chain molecule made up of many small molecules (monomers) that have joined together
addition polymerisation
a chemical reaction where the double bonds in the monomer molecuels break and they add on to each other
thermosoftening polymer
flexible polymer that melts when heated, can easily be recycled
thermosetting polymer
brittle polymer that doesn’t melt when heated as they have cross links.
covalent bonding is…
ionic bonding is…
metallic bonding…
non metals
metal + non metal
metals
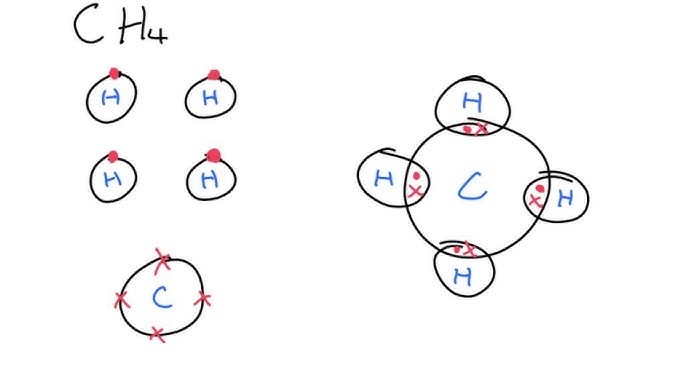
how do you represent covalent bonding
e.g CH4
dot and cross diagram
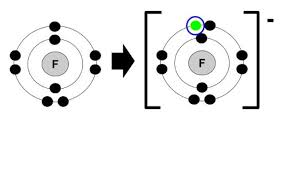
how do represent ionic bonding
e.g F
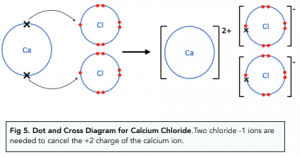
Ca Cl2
forming ions
making polymers from monomers
define exothermic reactions
and examples (chemical reactions (4)+ uses (2))
reactions that release thermal energy. These surroundings get hotter
examples
chemicla reactions- oxidation, combustion, neutralisation .displacemnet
uses- self heating cans, hand warmers
define endothermic reactions
and examples (chemical reactions(1) + uses (1))
reaction that absorb energy from the surroundings. Surroundings get colder
examples
chemical reactions- thermal decomposition
uses- single- use coolpacks for sports injuries
why do single-use cool packs get cold (3 marks)
-because it is an endothermic reaction
-that absorbs energy from the surroundings
-as ammonium nitrate in cool paco dissolves
-surrounding get colder (for 3 marker only)
define activation energy
the energy required for a chemical reaction to happen
the minimum amount of energy the reactant particles require in order to collide with each other and react
endothermic and exothermic reactions energy profiles
how do we know which energy profile is which
endothermic→ reactants have less energy than he products so the energy must have been absorbed
exothermic→ reactants are higher than the product so the energy must have been released
If an exothermic reaction occurs in a closed container, what would happen to the temperature inside the container?
The temperature would increase
If an endothermic reaction occurs in a closed container, what would happen to the temperature inside the container?
The temperature would decrease

Which of the above arrows indicates the energy change of the reaction?
A
The energy change of the reaction is the difference in energy between the reactants and the products.

Which of the above shows the activation energy?
B
The activation energy is the difference in energy between the reactants and the top of the curve.
combustion of ethane word equation and symbol equation
ethane + oxygen → carbon dioxide + water
2C2H6+7O2 → 6H2O + 4CO2
display formular of combustion of ethane
what are the steps to calculate bond energy (4)
count the number of bonds broken
work out energy needed to break bonds (times number of bonds by that bond and add with the breaking bonds)
work out energy to make bonds (times number of bonds by that bond and add with product bonds)
find out overall energy change (enerfy brocken- energy made, exothermic if neg)
Does breaking bonds release energy or require energy?
Is breaking bonds exothermic or endothermic?
Require energy
Endothermic
bond energy define
the amount of energy required to break one mole of a particular covalent bond.

What is the energy change of the above reaction?
count the number of bonds
times the number of bonds the bond energy
add the amount of energy for the products breaking, then the reactants forming
then work out energy needed to make these bonds (total energy(Kj/m) =energy brocken-energy formed )
exothermic→ neg
endothermic →pos
ANSWER: -114 Kj/m exothermic reaction
what is a cell
cells contain chemicals which react to produce electricity
what do cells contain
a simple cell can be made by connecting 2 different metals in a constant with an electrolyte

what is a battery
batteries consist of 2+ cells connected together in a series to provide a greater voltage

why can some cells only be used once
in non rechargeable cells and batteries the chemical reactions stop when one of the reactants has been used up
which type of battery is non rechargeable
alkaline batteries
how are some types of batteries rechargeable
rechargeable cells and batteries can be recharged because the chemical reactions are reversed when an external electrical ccurrent is supplied
whats different about the performance of rechargeable and non rechargeable batteries
non rechargeable batteries output gradually reduces as the chemicals in them get used up whereas rechargebable batteries have a consgtant output until just before they need recharging
pros and cons of a non rechargeble battery (5,5)
pros
-cheap
-lifespan in low current appliences e.g clocks is long
-doesnt need a powersource to recharge
-energy stored is higher
-can be stored for up to 10 years
cons
-lifespan in moblike phones is very short
-long term cost of buying is expensive
-performance gradually reduces as chemicals are uused up
-doesnt work in higher power devises e.g electric drills
-environment damage ot be thrown away is high due to large quantity
pros and cons of rechargeable battery ()
pros
-lifespan in low current appliences e.g clocks is high
-lifespan in mobile phones is long
-performance is constant until just before recharging
-works in high power devises e.g drills
cons
-expensive
-long term cost of buying, recharging ect is high
-need a power source to recharge
-less energy is stored
-can only be stored for 2-3 years
-high environmental damage when trowing away due to metal content
what affects the bvolayage of a cell (3)
The metals used for the two electrodes. The greater the difference in reactivity of the two metals, the greater the voltage will be.
The type and concentration of the electrolyte used.
The conditions, such as temperature.
how are some batteries non rechargeable
In non-rechargeable cells and batteries the chemical reactions stop when one of the reactants has been used up. These are the batteries usually used in smoke alarms and tv remotes.

What do we call the pieces of metal in an electrochemical cell?
electrodes
how do hydrogen fuel cells work
+half equations
-hydroghen gas is supplied as a fuel to the neg electrode
-it diffuses through the graphite electrode and reacts with hydroxide ions to form water (waste product) and provides a source of electrons to an external circuit
-oxygen gas is supplied to the pos electrode - cathode (opposite to electrolysis)
-it diffuses through the graphite and reacts to form hydroxide ions, accepting electrons from external circuit
half equations
2H2(g)+ 4OH- (aq) →4H2O(l) + 4e- ——> cathode
O2(g) + 2HO(l) +4e’→4OH- (aq) ——→anode
Which direction do the electrons flow in a hydrogen-oxygen fuel cell?
From the anode to the cathode
What is the overall reaction for a hydrogen oxygen fuel cell?
hydrogen + oxygen ➔ water
are the anode pos or neg
is the cathode pos or neg
is this the same or different to electorolysis
In fuel cells the anode (which is drawn on the left) is negative, while the cathode (drawn on the right) is positive.
This is the opposite way around to the anode and cathode in electrolysis, so be careful not to mix them up.
What are the electrodes in fuel cells made from?
Porous carbon
When hydrogen gas enters a fuel cell, it loses electrons to become hydrogen ions. Is the hydrogen gas oxidised or reduced?
Oxidised
What are the main advantages and disadvantages of hydrogen-oxygen fuel cells? (3,3)
pos
Hydrogen and oxygen are both renewable
Fuel cells last longer than batteries
The reaction doesn't produce any pollutants
neg
Hydrogen gas requires a large space to store
It requires energy to produce hydrogen
Hydrogen is highly flammable, so dangerous to store
what are fuel cells
a type of electrochemical cell - meaning they convert their chemical energy (fuel and oxygen) into electrycal energy that we can use to power things e.g hydrogen, oxygen fuel cell -forms water and electrical energy
whats the structure of a fuel cell
centre theres an electrolyte (solution ions can move through)
either side are the electrodes (made of porous carbon, has tiny holes and a catalyst to speed up the reaction) - neg electrode on the left (anode), pos on the right (cathode), connected by a wire on the top - allows electrodes to flow from the anode around to the cathode
on the outside of the electrons we have the anode and the cathode compartments,with an inlet at the top of each and an outlet at the bottom cathode - hydrogen enters the anode compartment, oxygen enters the cathode compartment, all the water and heat leaves out the cathode compartment
how does a fuel cell work
hydrogen comes in through the anode compartment, oxidised by the anode
electrons pass around the wire to the cathode and the hydrogen ions move through the electrolyte to the cathode
electrons and hydrogen ions then react with the oxygen coming from the cathode compartment, to create water, water leaves via cathode outlet
as a fuel eneters the cell, it becomes oxidsed and this sets up a potential difference across the cell to generate electricity
What is the purpose of the temperate changes practical?
to investigate the variables that affect the temperature change in chemical reactions
2 hazards and risks and control measures to the temperate changes practical
(Hazard) copper sulfate is corrosive to eyes and irritant to skin (risk) damage to eyes and irritation to skin (control) wear safely spectacles and clear spills immediately, wash hands after practicle
(hazard) stirring and taking temp of a solution in a polystrene cup (risk) piercing the cup/tipping over- leads to spillage (control) place cup in a glass beaker for stability and to contain spills
CID variables for temp changes rp
control
-mass of zinc
-volume of copper sulfate
independant
-concentration of copper sulfate
dependant
-temp change
method for temp changes rp
put 10cm3 copper sulfate solution into 50cm3measuring cylinder
top measuring cylinder up to 50 with water
transfer diluted copper sulfate solution to a polystrene cup inside the glass beaker and use the thermometer to measure temp. record initial temp
use the balance, measure 1g of zinc powder on scrap paper
add zinc powder to the cup and stir vigorously
continue stirring until reading on themometer stops changing, write final temp in results table
repeat steps for different dilutions of copper sulfate, ensuring the total volume is 50 each time
calculate temp change for each experiment
plot a graph
how do you ensure you measured the temp of the solution accurately
looking at eye level and stiring vigourously
how do yuou measure the volume of the solution accurately
i looked at eye level and used a measuring cylinder
how could you ceck the repeatablity of your results
why would this improve the repeatablilty of your investigation
we could repeatability 3x and find a mean so any anomalies are ignored
what are the benefits of looking at other groups results
to see if your results are accurate and therefore repeatable
What is the purpose of the polystyrene cup and lid?
to prevent heat loss to the surroundings
reactivity
how easily electrons are lost/gained
(nothing to do with melting/boiling)
oxidation
process of gaining oxygen
e.g 2mg + O(2) → 2MgO
in electrolosys- loss of electrons
reduction
the loss of oxygen
e.g 2MgO →2Mg+O(2)
in electrolosys- gaining electrons
displacement reaction
where a more reactive metal displaces a less reactive metal from its compound
what do the roman numerals show on the transition metals
eg. Iron (II) chloride
to show the charge of transition metals
FeCl(2)
why is potassium more reactive than lithium (3)
-atoms get bigger down the group
-less attraction between the nucleus and the outer electron
-easier to loose an electron so more reactive (opposite for group7)
how do we explain the different reactivity’s of metals
-for a metal atom to react it must loose electrons to become a postitive ion.
-the easier an atom can do this,the more reactive the more reactive the metal will be
reactivity series
potassium (k)
sodium (Na)
Lithium (Li)
Calcium (Ca)
Magnesium (Mg)
Aluminium (Al)
Carbon (C)
Zinc (Zn)
Iron (Fe)
Hydrogen (H)
Copper (Cu)
Silver (Ag)
Gold (Au)
ore
a rock containing an ionic metal,compound,usually a metal oxide
how is iron extracted
-reduction with carbon
-carbon and iron ore (iron oxide) are heated to a very high temp in a blast furnace →iron is reduced- iron oxide + carbon →iron +carbon dioxide
-very wasteful
-bioleaching + phytomining produce solutions containing copper
-so you have to do electrolisis or displacement with scrapirion
how does pytomining work (4)
copper ions in soil are abdorbed by plants roots
copper ions become part of the plant
plant is burned-copper ions join with oxide ions in the air (forms copper oxide, CuO)
ash containing copper oxide reacts with sulfuric acid then filtered-makes copper sulfate solution- ash is dissolved -electrolysis of cop sul solution copper metal collects at neg electrode
advantages(6) and disadvantages(6) of phytomining
advantages
-uses low grade copper ore
-less energy than smelting
-less air polution produced
-less waste rock
-decontaminates polluted ground
-produces less greenhouse gases then smelting
disadvantages
-can produce toxic chrmicals
-a lot slower
-electrolysis requiress lots of energy (and money)
-plants need good growing conditions
-don’t get much-lower yield
-dependent on weather
how does bioleaching work (3)
-bacteria are used to extract copper ions from low grade copper ores
-bacteria converts copper compounds within ores into solution
-these copper compound solutions can be separated with electrolysis or displacement reactions to form copper metal
advantages and disadvantages of bioleaching
advantages
-economical→simpler,cheaper
-environmental→less landscape damage- bacteria grows naturally
-ore conservation→can eextract metals from ores that are too poor for other technologies
-low grade ores
-produces less air pollution than smelting
disadvantages
-economical-even slower compared to smelting-less profit
-environmental-toxic chemicals are produced →heavy metal ions leak during acid mine drainage
pros/cons of smlelting (1,2)
pro
-have equiptment already/know how it works
cons
-lots of equiptment is used
-produces lots of CO(2)