Chapter 4:Molecular compounds
The bond formed when atoms share electrons is called a covalent bond, and the group of atoms held together by covalent bonds is called a molecule.
Although metals and reactive non-metals can achieve an electron octet by gaining or losing an appropriate number of electrons to form ions, non-metals can also achieve an electron octet by ==sharing an appropriate number of electrons in covalent bonds.==
The electrons act as a kind of “glue” to bind the two nuclei of atoms together into a molecule. Both nuclei are simultaneously attracted to the same electrons and are held together.
This optimum distance between nuclei is called the bond length .Typically, the bond length is ==slightly less than the sum of the atomic radii of the two atoms involved in the covalent bond.==
Covalent bonds can form between unlike atoms as well as between like atoms, making possible a vast number of molecular compounds
A bond formed by sharing two electrons (one pair) is a single bond, a bond formed by sharing four electrons (two pairs) is a double bond, and a bond formed by sharing six electrons (three pairs) is a triple bond.
Sharing more than two electrons ==increases the attractive forces between the two atoms== and pulls them closer together. Hence, the bond length decreases in the order single bond>double bond>triple bond.
Sometimes, a bond is formed by the overlap of a filled orbital on one atom with a vacant orbital on another atom ==so that both electrons come from the same atom==. The bond that results in this case is called a coordinate covalent bond.
Formation of a coordinate covalent bond often results in unusual bonding patterns, such as an N atom with four covalent bonds rather than the usual three, or an oxygen atom with three bonds rather than the usual two.
When intermolecular forces are very weak, molecules of a substance are so ^^weakly attracted^^ to one another that the substance is a gas at ordinary temperatures. If the forces are ^^somewhat stronger^^, the molecules are pulled together into a liquid; and if the forces are ^^still stronger^^, the substance becomes a molecular solid.
- Even so, the melting points and boiling points of molecular solids are usually lower than those of ionic solids because the ==intermolecular forces between molecules are weaker than the electrostatic attractive forces between ions.==
Most molecular compounds are insoluble in water, because they have little attraction to the strongly polar water molecules.
Molecular compounds, generally, do not conduct electricity when melted because they have no charged particles.
Molecular formulas are formulas that show the number of atoms and type on atoms in one molecule of the compound.
Structural formulas, use lines to show how atoms are connected, and Lewis structures, show both the connections among atoms and the placement of unshared valence electrons.
A molecular formula %%gives the number of atoms that are combined in one molecule%% of a compound, whereas an ionic formula gives only %%a ratio of ions.%%
Steps for drawing a lewis structure:
STEP 1: Find the total number of valence electrons of all atoms in the molecule or ion.
taking an example of PCl3, phosphorus (group 5A) has five valence electrons and chlorine (group 7A) has seven valence electrons, giving a total of 26.
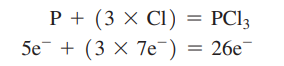
STEP 2: Draw a line between each pair of connected atoms to represent the two electrons in a covalent bond.
the P atom is in the centre and the Cl atoms are bonded to it.
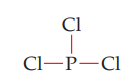
STEP 3: Using the remaining electrons, add lone pairs so that each atom connected to the central atom (except H) gets an octet.
In PCl3, six of the 26 valence electrons were used to make the covalent bonds. From the remaining 20 electrons, each Cl atom needs three lone pairs to complete the octet.
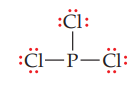
STEP 4: Place any remaining electrons in lone pairs on the central atom.
In PCl3, we have used 24 of the 26 available electrons—six in three single bonds and 18 in the three lone pairs on each chlorine atom. This leaves two electrons for one lone pair on phosphorus.
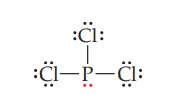
STEP 5: If the central atom does not yet have an octet after all electrons have been assigned, take a lone pair from a neighbouring atom and form a multiple bond to the central atom.
In PCl3, each atom has an octet, all 26 available electrons have been used, and the Lewis structure is finished.
Molecular shapes can be predicted by noting how many bonds and electron pairs surround individual atoms and applying the valence-shell electron-pair repulsion (VSEPR) model.
There are three steps to applying the VSEPR model:
- STEP 1: Draw a Lewis structure of the molecule, and identify the atom whose geometry is of interest.
- STEP 2: Count the number of electron charge clouds surrounding the atom of interest. The number of charge clouds is simply the total number of lone pairs plus connections to other atoms.
- STEP 3: Predict molecular shape by assuming that the charge clouds orient in space so that they are as far away from one another as possible.
When the atoms in a compound are not identical, the ^^bonding electrons are attracted more strongly by one atom than by the other and are shared unequally^^. Such bonds are said to be polar covalent bonds.
The ability of an atom to attract electrons in a covalent bond is called the atom‘s electronegativity.
Fluorine is the most electronegative element.
Metallic elements on the left side of the periodic table attract electrons only weakly and have lower electronegativities, whereas the %%halogens and other reactive non-metal%% elements %%attract electrons strongly and have higher electronegativities.%%
The partial charges associated with each end of the bond result in a dipole. @@The larger the dipole associated with a bond, the more polar it is.@@
Molecules can be polar if electrons are attracted more @@strongly to one part of the molecule than to another.@@ Molecular polarity is due to the sum of all individual bond polarities and lone-pair contributions in the molecule.
When two different elements combine, they form a binary compound.
Naming a binary compound:
- STEP 1: Name the first element in the formula, using a prefix if needed to indicate the number of atoms.
- STEP 2: Name the second element in the formula, and modify by adding the -ide suffix as when naming anions.