Biochem Week 2
1/105
Earn XP
Description and Tags
Glucose Metabolism, Pentose Phosphates, Maintaining Blood Glucose
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
106 Terms
major dietary carbohydrate
glucose
what structures depend on glucose particularly as fuel
brain (75% of daily intake), red cells, muscle
glucose is metabolized by what process
glycolysis
other carbohydrates (fructose, galactose, mannose) are metabolized by
conversion to glycolytic intermediates
glycolysis is the conversion of glucose to _______ (aerobic) or ________ (anaerobic)
pyruvate (aerobic)
lactate (anaerobic)
differences between hexokinases and glucokinases
hexokinases can phosphorylate other hexoses, while glucokinase is specific to glucose.
hexokinases are ubiquitous, while glucokinase is restricted to liver and pancreatic beta cells.
hexokinases are inhibited by G6P (glucokinase is not)
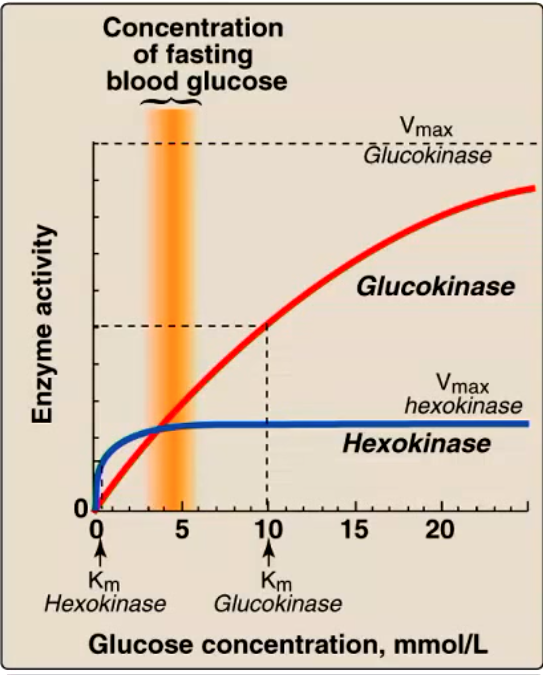
what does this figure tell us about the functions of hexokinase and glucokinase?
hexokinase Km = 0.1 mM (way below normal blood glucose of 5 mM, always working as hard as it can, fully saturated)
glucokinase Km = 10mM (becomes much more active after eating)
if hexokinase is always active, how is it regulated so that it’s not constantly stealing glucose from the circulation?
inhibit the byproduct (G6P) (cells that have a lot of G6P means they have metabolized all they need/don’t need more)
why does it make sense that glucokinase is not inhibited by G6P?
we want glucokinase to work in the 2 places that have it (liver and pancreas) to continue functioning even in presence of glucose
liver brings down glucose concentration AND metabolize it for energy
pancreas
some people become diabetic even though they have pancreatic beta cells that make insulin. what type of diabetes is this? what’s it called when it occurs in younger people?
type 2
mature onset diabetes in the young (MODY)
MODY (mature onset diabetes in the young)
specific type of diabetes type 2
based on single mutation in one of several genes (commonly mutation in glucokinased)
how does glucokinase deficiency cause MODY?
glucokinase activity regulates rate of glycolysis that in turn regulates insulin secretion
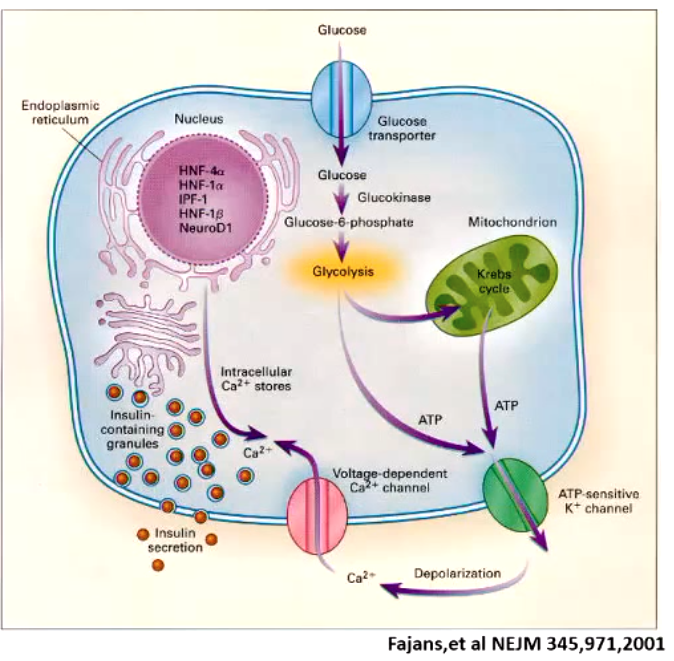
hypoglycemia can result in
coma
red blood cells don’t have mitochondria. so what do they depend on?
can’t do electron transport/oxidative phosphorylation (processes where most of energy for glycolysis)
rbc get ALL energy from glycolysis instead
______ provides muscle the energy for exercise
glycolysis
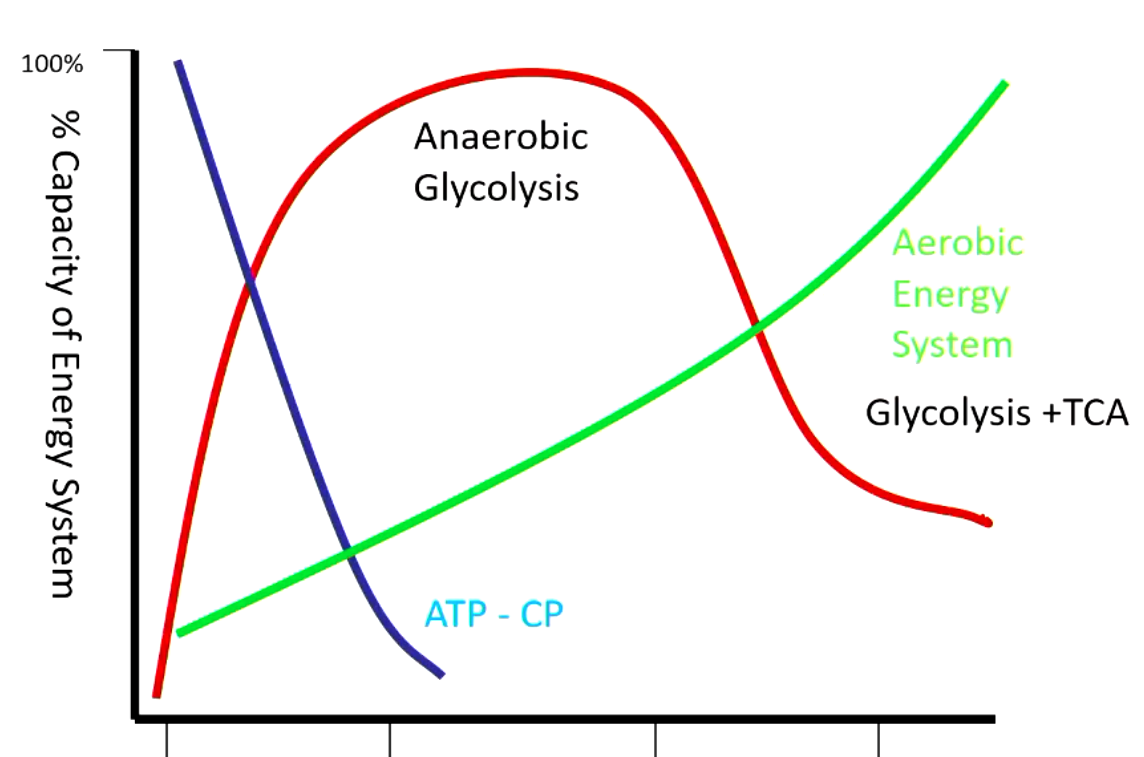
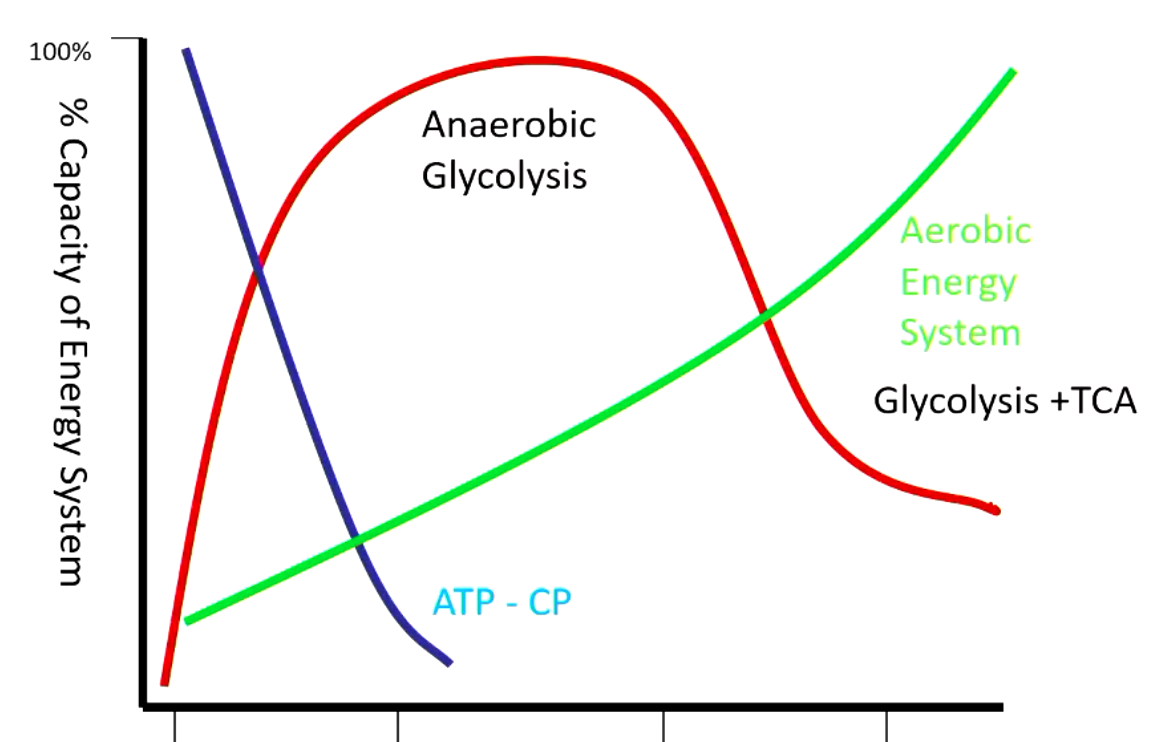
very beginning - using energy in form of ATP or creatine phosphate (depleted within seconds)
next period - completely dependent on anaerobic glycolysis (muscle is not oxygenated yet)
after - aerobic glycolysis + TCA
in both aerobic and anaerobic glycolysis, each glucose molecule will produce
2 ATP (directly)
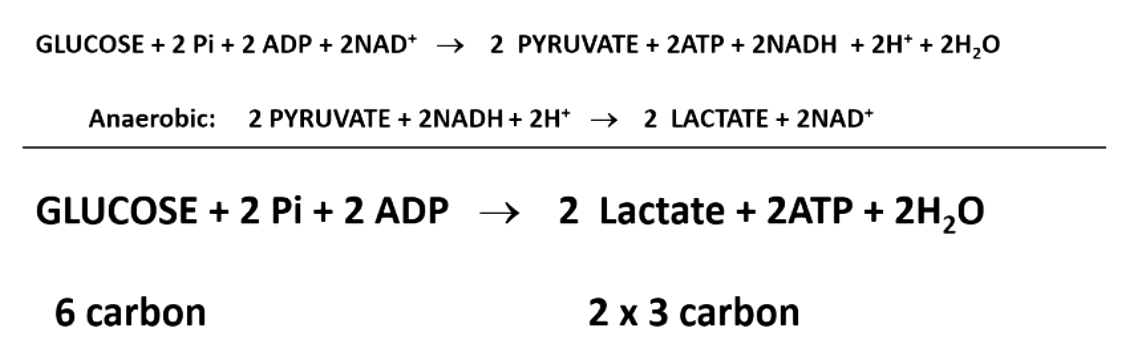
the first phase of glycolysis is called the investment phase because
uses 2 ATPs to generate 2 phosphorylated 3-carbon intermediates
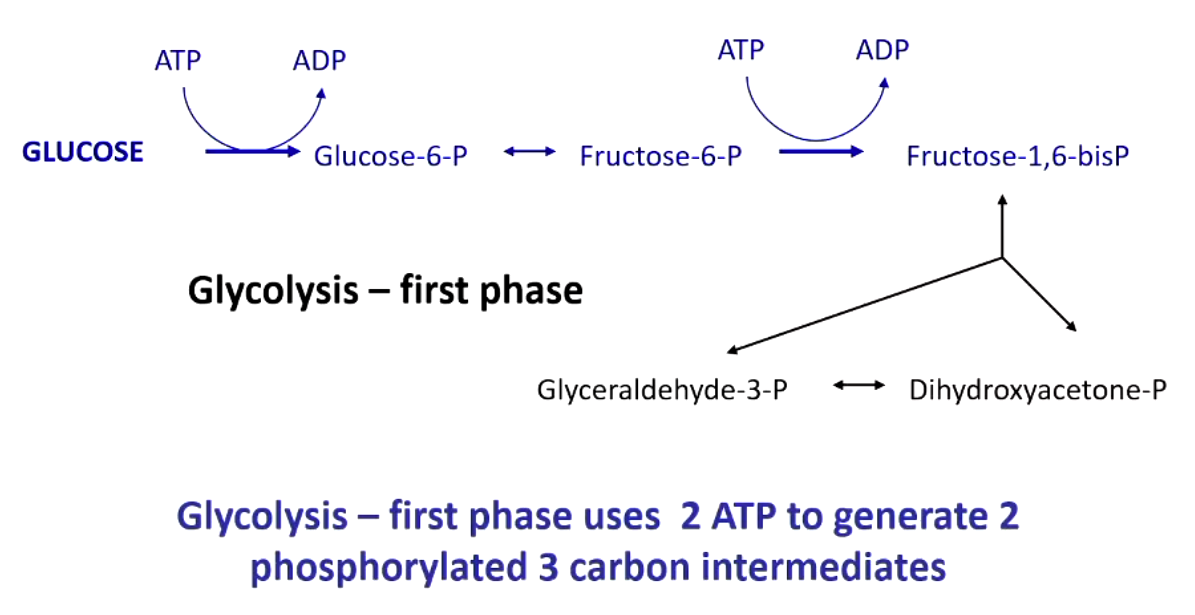
to make ATP, you need to generate
high energy phosphate
glyceraldehyde 3-phosphate dehydrogenase requires
inorganic phosphate AND coenzyme NAD+
glyceraldehyde 3-phosphate dehydrogenase shows
negative cooperativity (insulates enzymes from changes in substrate concentration)
NAD+ (nicotinamide adenine dinucleotide) comes from _______. what does a deficiency in this cause?
niacin (vitamin B3)
D3 (diarrhea, dermatitis, dementia)
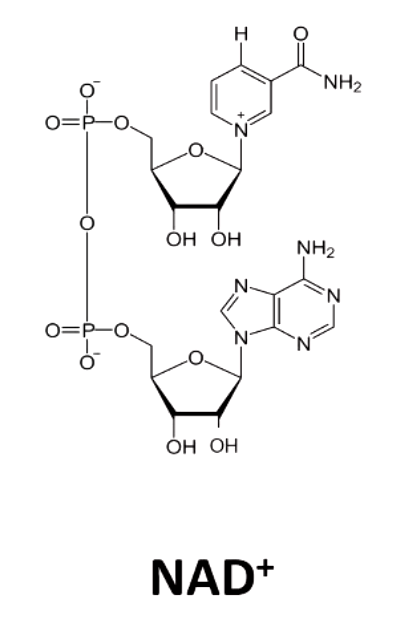
NAD + accepts ____ from substrate
2 electrons and 1 hydrogen
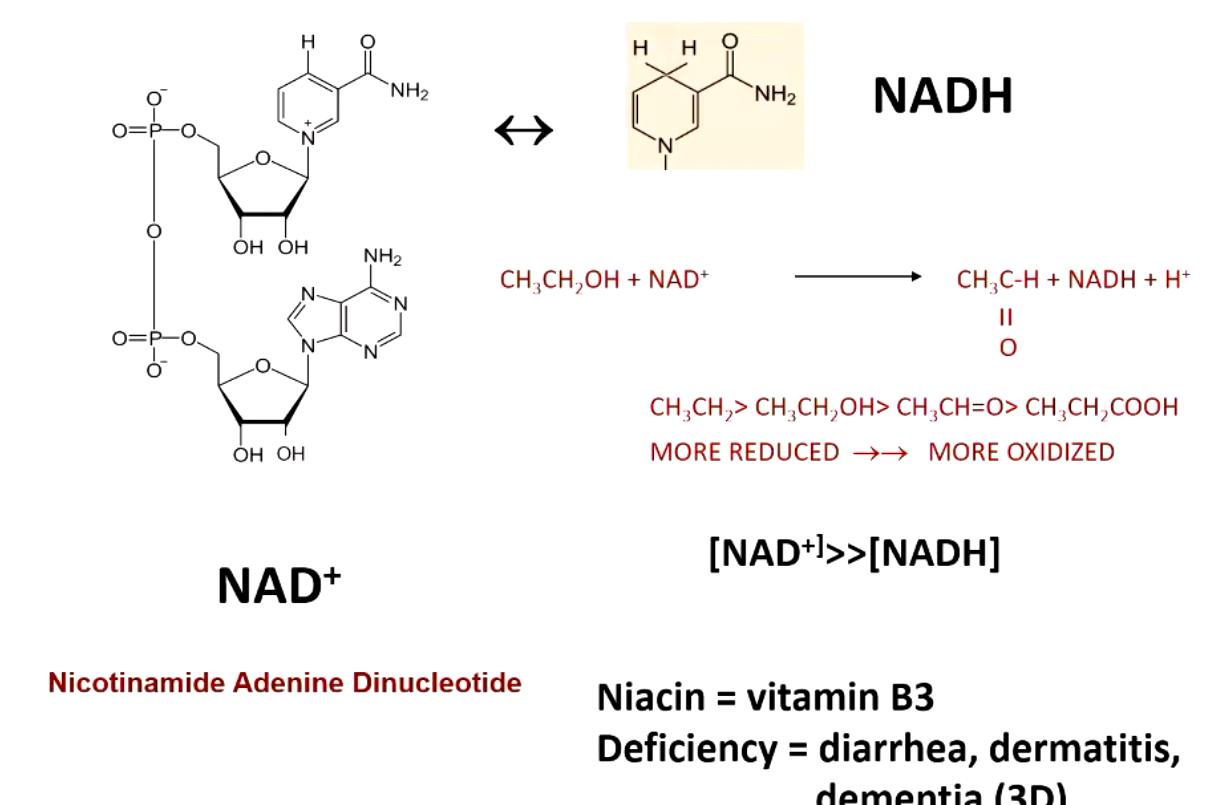
what happens when NAD+ is low (anaerobic conditions)?
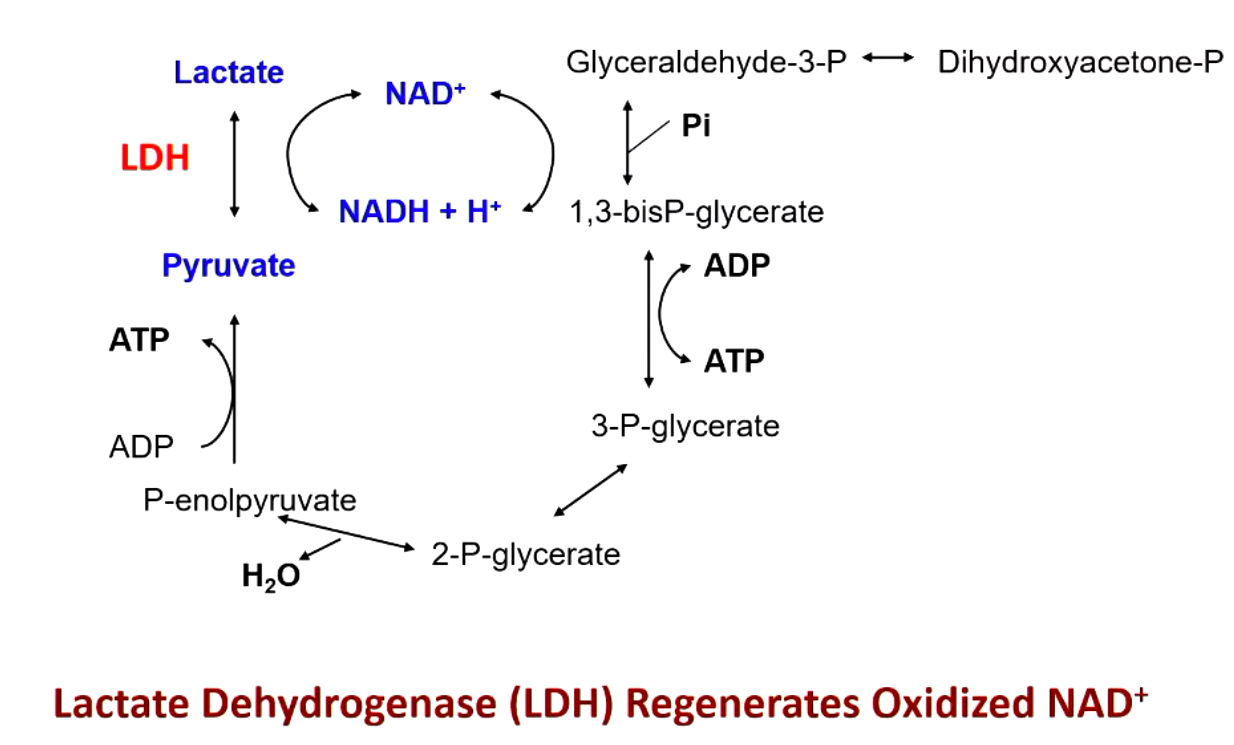
lactate dehydrogenase (LDG) regenerates oxidized NAD+
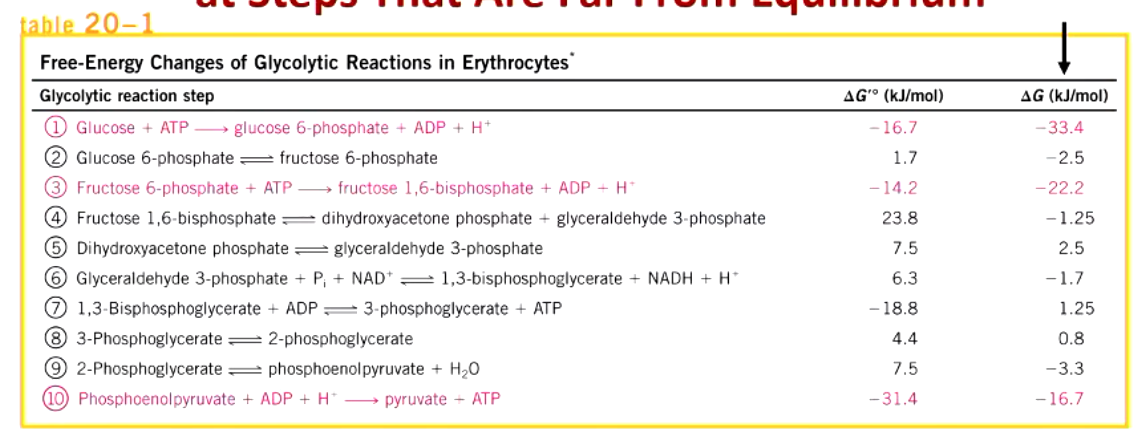
regulation of glycolysis occurs at steps far from equilibrium. what steps are they (3)?
hexokinase
phosphofructokinase
pyruvate kinase
phosphofructokinase does what (inhibited by? activated by?)
key regulated step in glycolysis (converts fructose-6-P to F1,6-BisP)
inhibited by intracellular ATP and citrate; activated by AMP
key positive regulator=fructose-2,6-bisphosphate
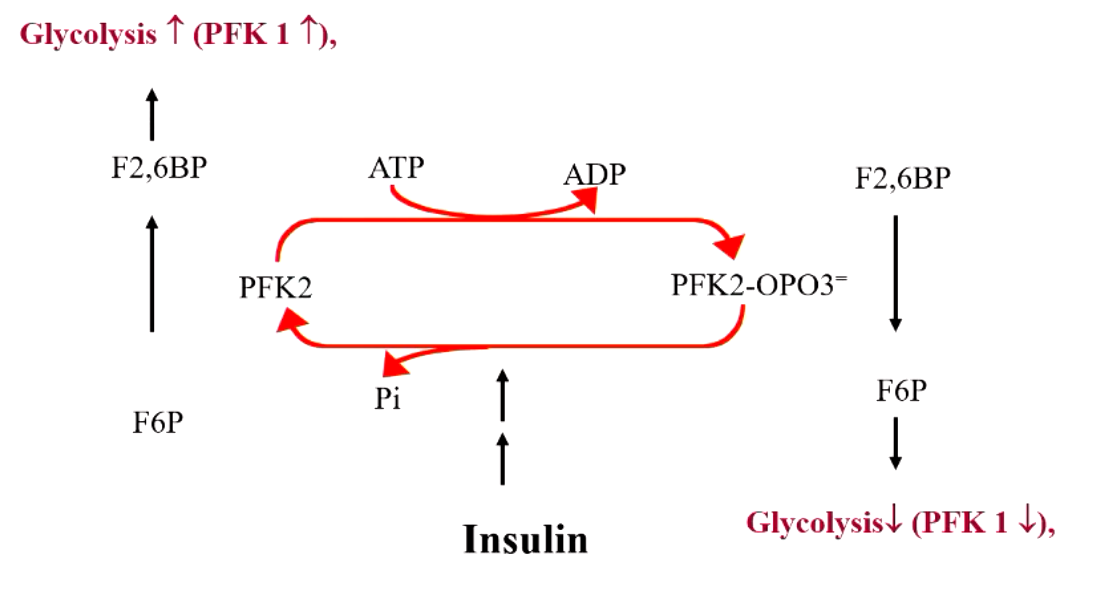
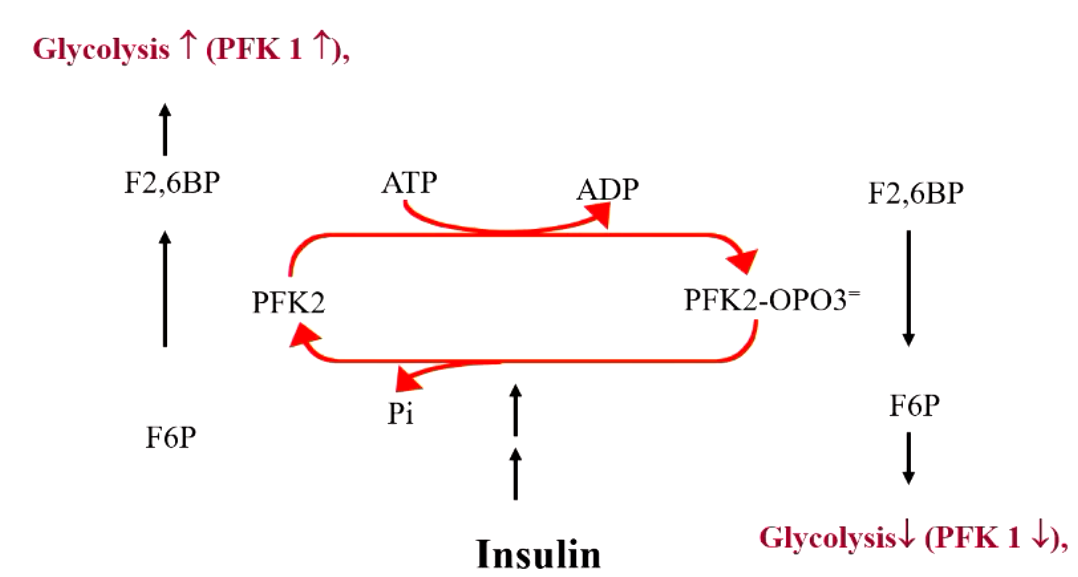
explain what’s happening here
F6P + PFK1 → F-1,6-BisP
F6P + PFK2 → F-2,6-BisP (this is a positive regulator for F-1,6-BisP, increase PFK1 activity = increased glycolysis)
but if PFK2 is phosphorylated, it turns into a phosphate that DEGRADES F-2,6-BisP (which then decreases PFK1 activity = decreased glycolysis)
explain the effects of insulin vs glucagon on PFK2 “seesaw”
insulin drives to left (glycolysis increase)
glucagon drives to right (decreased glycolysis)
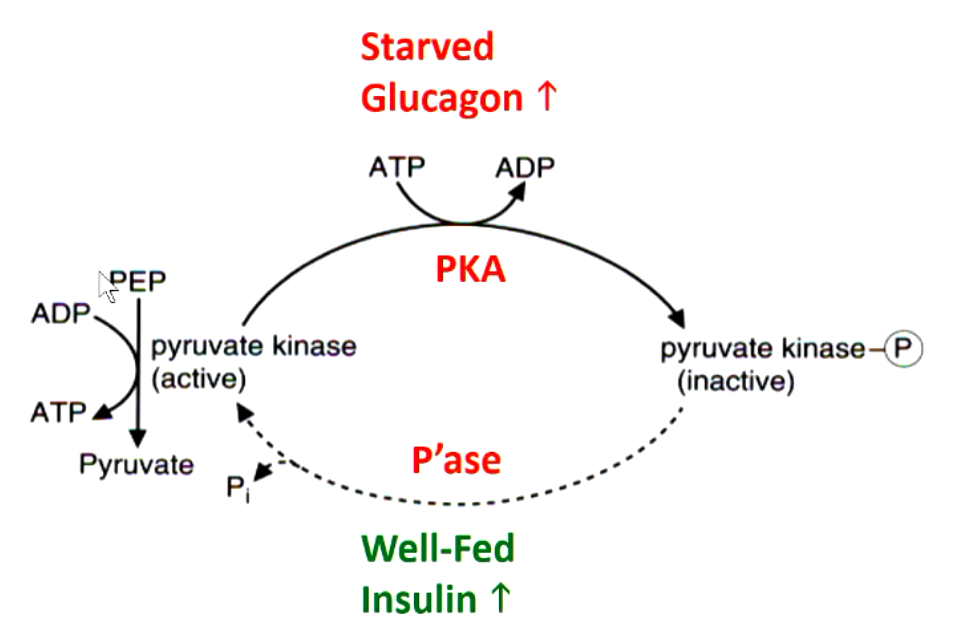
how is pyruvate kinase regulated in the liver
protein kinase A (PKA) enzyme phosphorylates pyruvate kinase and turns it inactive (in response to glucagon)
phosphatase enzyme dephosphorylates inactive pyurvate kinase into active form (in response to insulin)
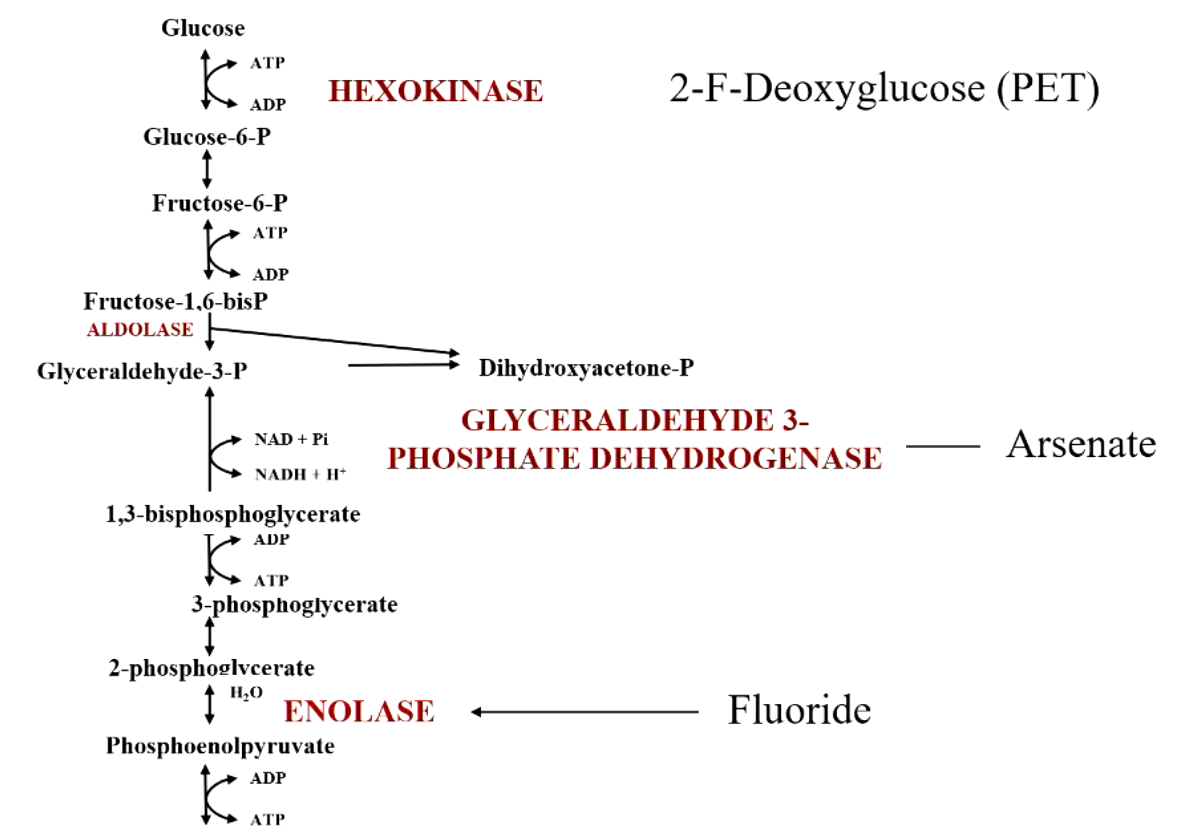
certain enzymes in glycolysis are targets of toxic chemicals. what are they/
hexokinase - 2-F-deoxyglucose (PET)
glyceraldehyde 3-phosphate dehydrogenase - arsenate
enolase - fluoride
PET imaging can be used to detect
metastasis (tumors have increased glycolysis rate)
chemotherapy progress
fluoride is generally thought to be good. why?
affect bacterial metabolism
inhibit demineralization
enhance remineralization
too much fluoride causes
fluorosis
38 yo woman anemic since birth. 150 blood transfusions, high number of immature red blood cells. rbc have short life. no response to medication. what’s defective?
defective RBC pyruvate kinase
pyruvate kinase comes in 4 isozymes: M1, M2, L, and R (only R is defective in this case so all other tissues such as liver are normal)
3 adults siblings okay at rest but easily fatigued. can’t keep pace with others, weakness/stiffness in exercise. no rise in blood lactate in exercise. what’s defective? and why aren’t they anemic?
defective muscle PFK I (3 forms of PFk isozymes. muscle has one specific form while RBCs have mix of 3)
can’t convert glucose under anaerobic conditions
glycolytic enzymes that catalyze 3 irreversible steps are sites of regulation. what are they?
glucokinase/hexokinase (beta cells)
phosphofructokinase (many tissues)
pyruvate kinase (liver)
intermediates of glycolysis produce important metabolites like
glycogen, ribose, NADPH (from G6P)
glucosamine (from F6P)
trigylcerides, phospholipids (from dihydroxyacetone)
2,3-bisphosphoglycerate red blood cells (from 1,3-bisphosphoglcerate)
serine (from 3-phosphoglycerate)
alanine (from pyruvate)
glycolysis occurs in the ________ and requires
cytoplasm
Pi and NAD+
2 important products of pentose phosphate pathway
ribose-5-phosphate (nucleotide synthesis)
NADPH (reduce glutathione, synthesize fatty acids, detoxify drugs, etc.
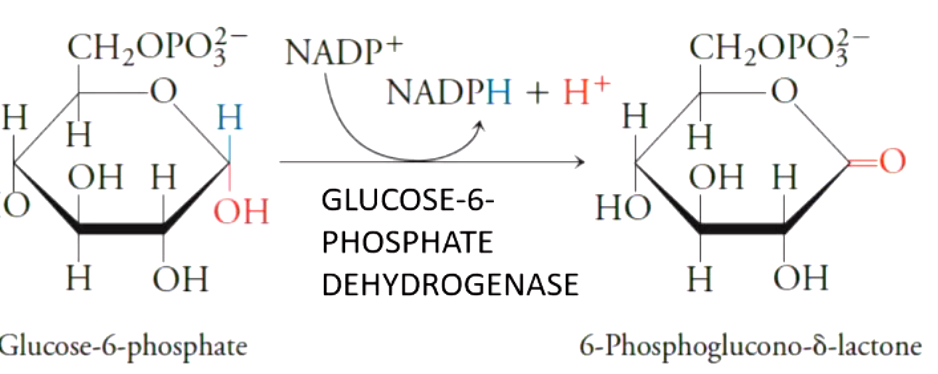
first oxidative portion of pentose phosphate pathway produces
2 NADPHs while converting G6P to pentose ribulose 5 phosphate and CO2
the first key enzyme of the pentose phosphate pathways is
glucose 6 phosphate dehydrogenase (key regulated step)
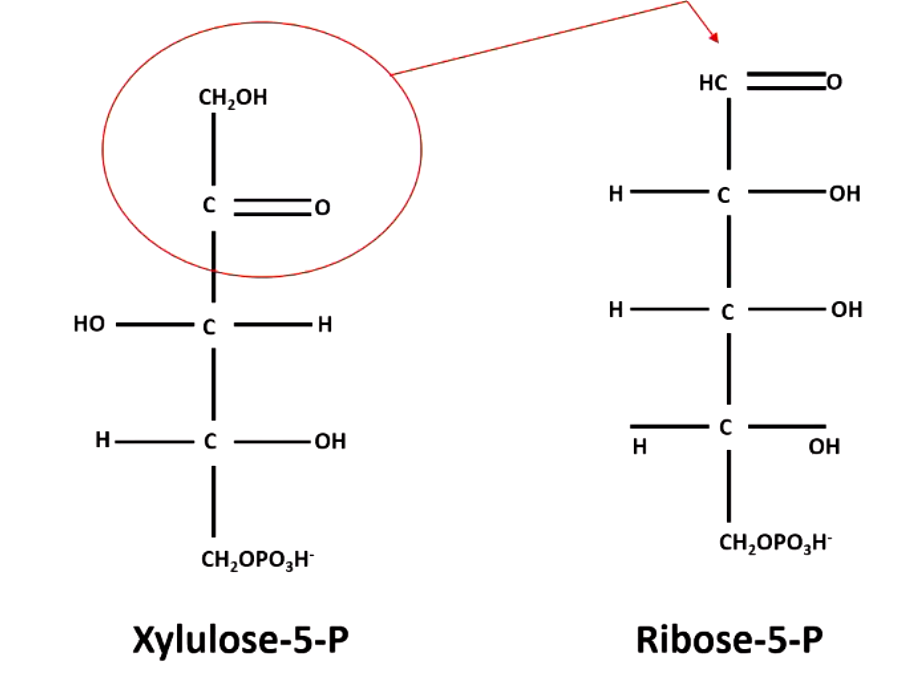
transketolose transfer __ carbons using thiamine
2 carbons
thiamine deficiency causes
wet or dry beriberi
wernicke-korsakoff (alcoholics)
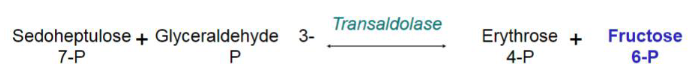
transaldolase transfer __ carbon unit
3
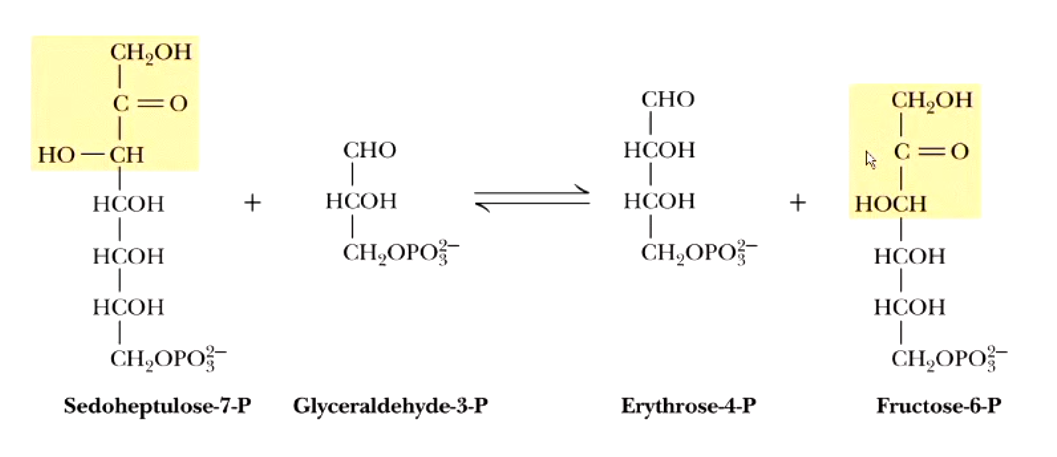
transketolase takes a+b —> c+d
xylulose 5-P + ribose 5-P → sedoheptulose 7-P + glyceraldehyde 3-P
AND
xylulose 5-P + erthyrose 4-P → glyceraldehyde 3-P + fructose 6-P
the non-oxidative phase of pentose phosphate pathway ultimately takes 3 pentose-P and turns them into
1 triose-P (glyceraldehyde 3-P) + 2 hexose P (2 fructose 6-P)
transaldolase takes a+b —> c+d
glucose-6-P dehydrogenase is the first committed step (rate-limiting) of the pentose phosphate pathway. it is inducible by? inhibited by?
insulin
NADPH
reduction of oxygen yields
highly reactive species
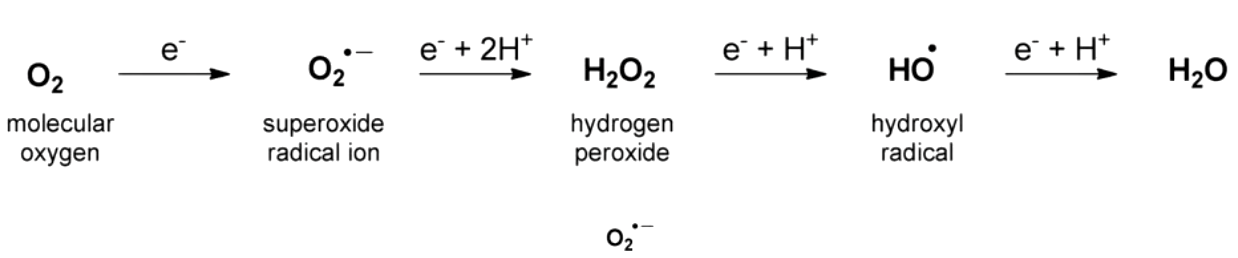
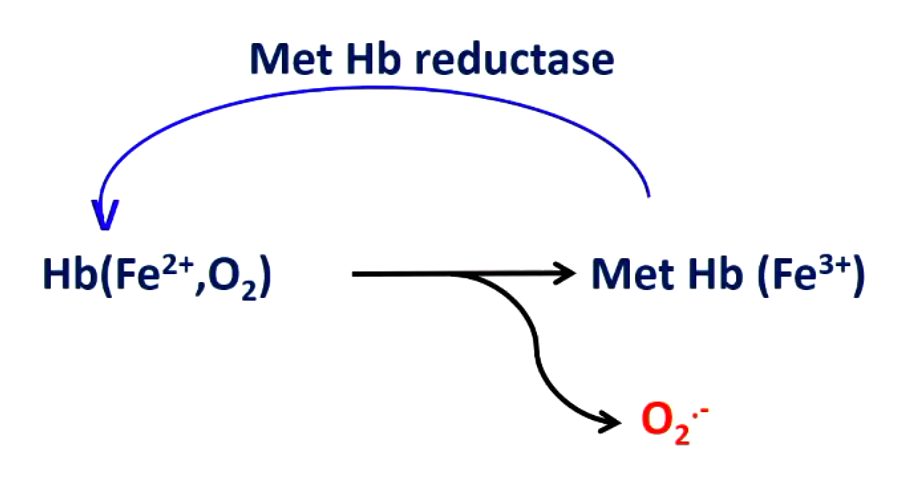
iron of hemoglobin and O2 react to give __________. what enzyme deals with this issue?
superoxide (Fe3+ doesn’t carry oxygen which would be dead hemoglobin
hemoglobin reductase (take Fe3+ and turn it back into Fe2+)
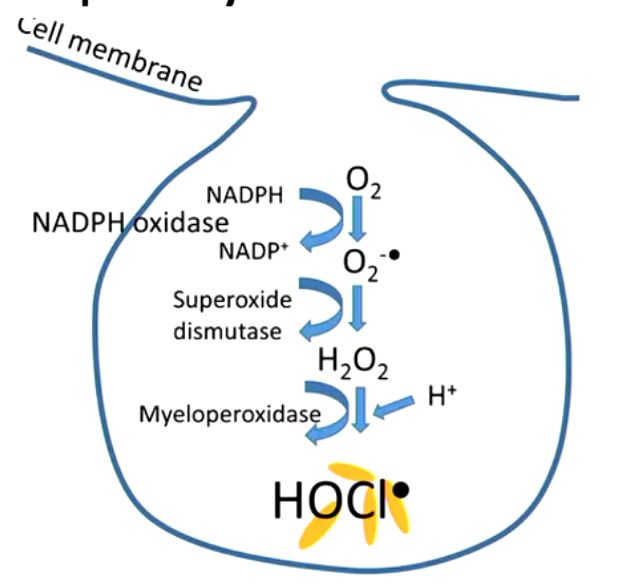
when is generation of radicals good?
respiratory burst in neutrophils (generate species that kill bacteria/viruses, fight infections)
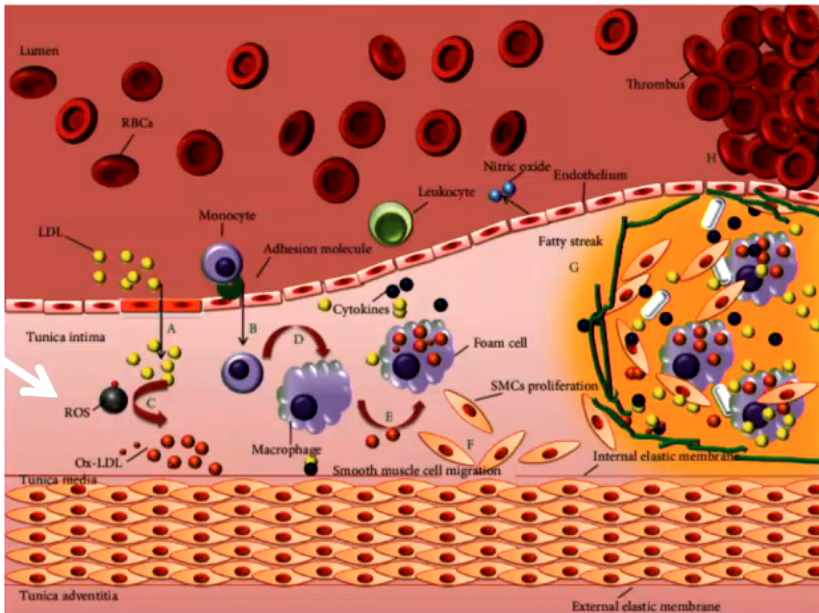
free radical injury is important in many disease states like
atherogenesis (oxidation of LDL)
ischemia
alcoholism
regenerative disease (ALS)
acute renal failure
emphysema
what can inactivate radicals?
antioxidants (vitamins A, C, E
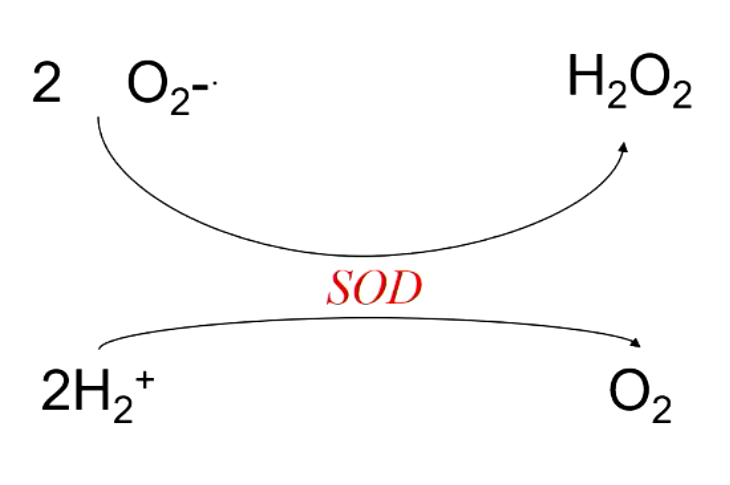
what enzyme converts superoxide into hydrogen peroxide (protection from radicals)?
superoxide dismutase (SOD)
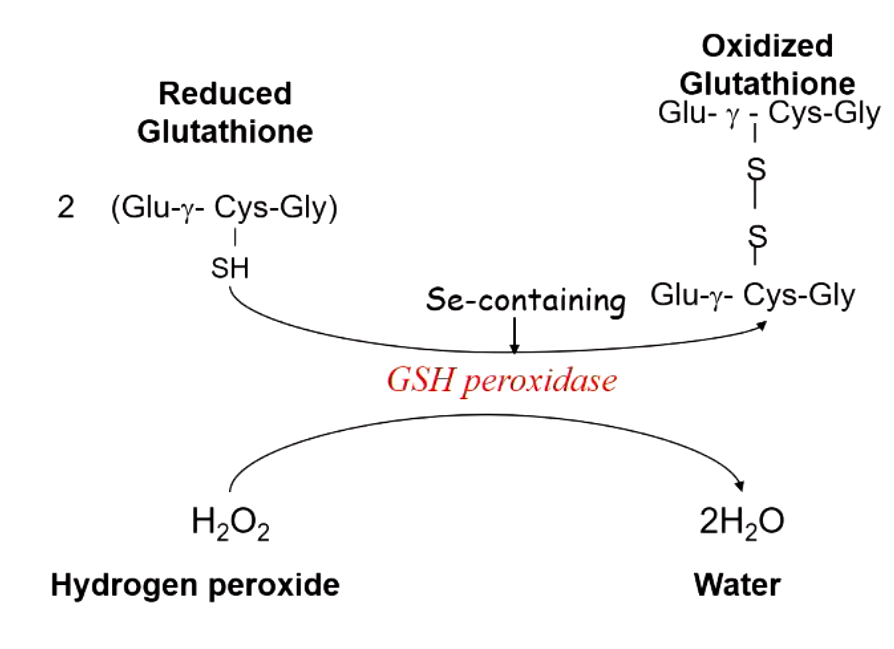
what is required for reduction of peroxides by peroxidase? what maintains the supply of this?
glutathione (GSH) which contains selenium
G6P dehydrogenase produces NADPH which maintains glutathione levels needed to destroy peroxide
young man with malaria is treated with primaquine. after several days, he presents with dark colored urine, low RBC count, elevated reticulocyte count, low hemoglobin, elevated bilirubin. what’s happening?
G6P dehydrogenase deficiency (extremely common)
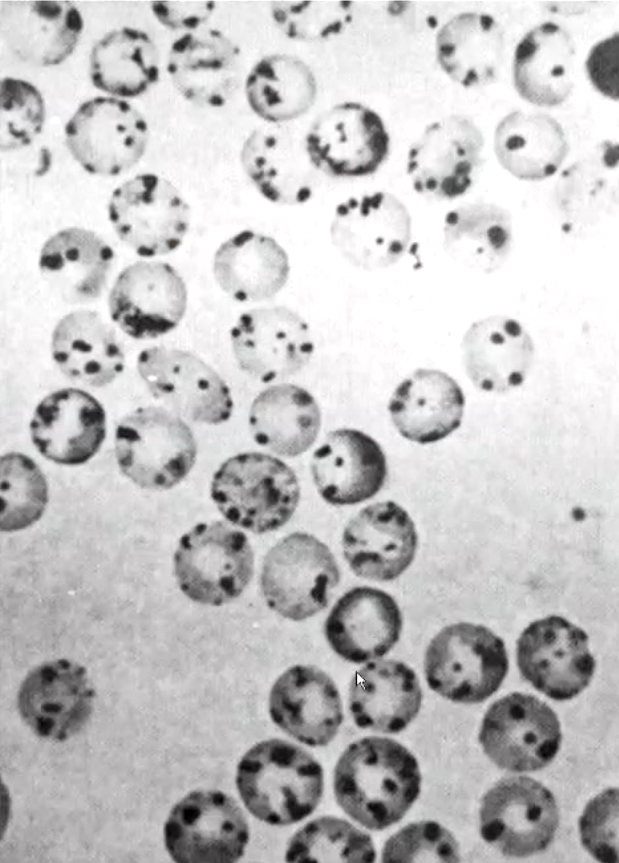
what’s being demonstrated?
G6PDH deficinecy (“Heniz bodies” form in RBC when cells exposed to oxidizing chemical in vitro
precepitiation of hemoglobin occurs due to disulfide bond formation between Hb molecules
some drugs/foods cause hemolytic anemia in some people. what are some examples
sulfonamides, aspirin/NSAIDs, quinadine/quinine, mothballs, fava beans
why are RBCs particularly sensitive to oxidative damage?
other cells have alternative pathways to produce NADPH (eg. malic enzyme)
without adequate glucose, there are 2 types of effects:
adrenergic (autonomic) = level 1 (glucose <70 mg/dL
trembling, palpitation, sweating, anxiety, nausea, hunger, tingling
neuroglycopenic = level 2 (glucose < 54 mg/dL)
headache, confusion, weakness, drowsiness, vision changes, difficulty speaking, dizziness, tiredness
what should u do if u have a patient with mild/moderate hypoglycemia?
give patient anything containing sugar (orange juice, hard candy)
get medical assistance
what should u do if u have a patient with severe hypoglycemia?
give IV 50% dextrose or 1 mg glucagon IM
summon medical assistance
blood glucose is hormonally maintained by (3 main)
insulin, glucagon, epinephrine (stress hormone)
glucagon vs insulin (made where? signal of what?)
glucagon - pancreatic alpha cells, signal of fasting
insulin - pancreatic beta cells, signal of feeding
glucagon vs insulin (chain length?)
glucagon - single aminoacid chain (29 aminoacids)
insulin - 2 aminoacid chains
glucagon vs insulin (target tissues? target metabolism?)
glucagon - liver, adipose (NOT MUSCLE) + carbs/lipids
insulin - liver, adipose, skeletal muscle + carbs/lipids/proteins
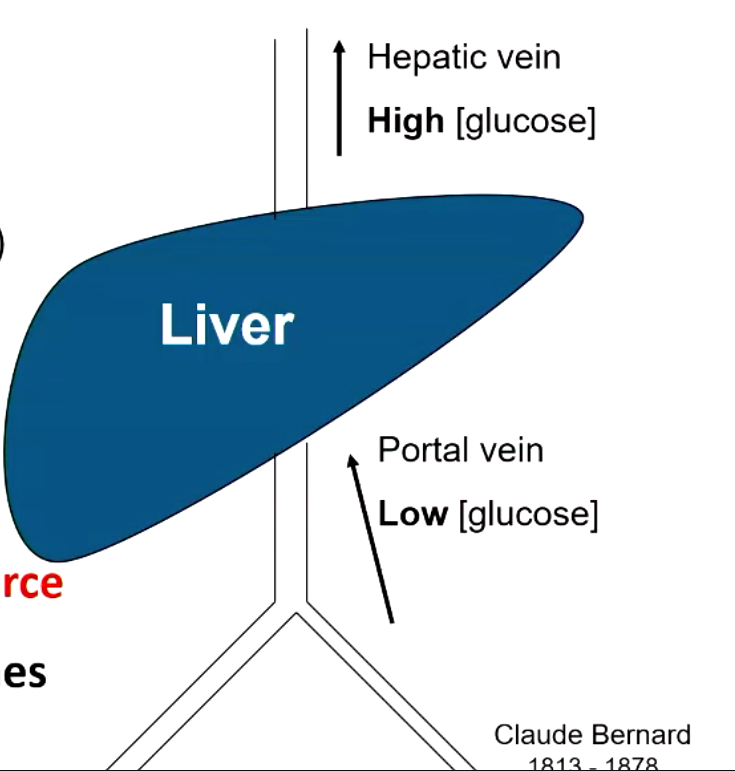
which organ is the major source of glucose?
liver
describe Claude Bernard’s experiment
his dogs do not eat carbs so blood entering liver had low glucose concentration while blood leaving liver had high glucose concentration
proved that liver is major source of glucose
liver has 2 sources of glucose. they are
glycogen: stored poly-glucose (sustains blood glucose for a few hours after a meal)
gluconeogenesis: de novo glucose synthesis (sustains blood glucose for many days/weeks in absence of carb intake)
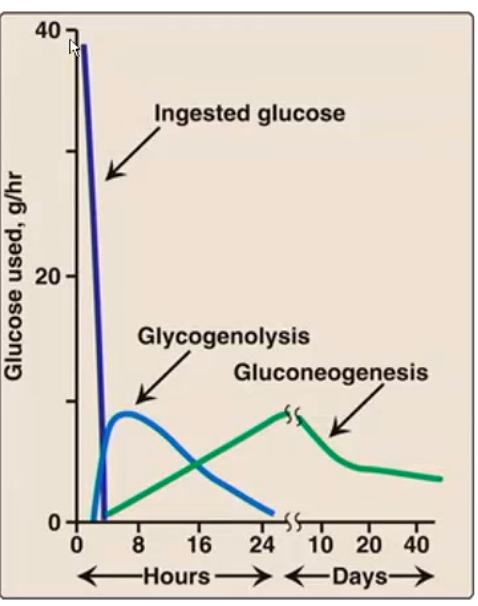
cross-over of glycogenolysis and gluconeogenesis (2 sources of glycogen from liver) occurs __ hours after eating
glycogen becomes unimportant after
16 hours
1 day
why bother making glycogen?
glucose transport is facilitated, so a high concentration would leak out
high glucose would pose major osmotic problem (diarrhea)
high glucose would glycate proteins
glucose reacts with metals to give ROS
where do we find glycogen?
liver (100g)
muscle (400g)
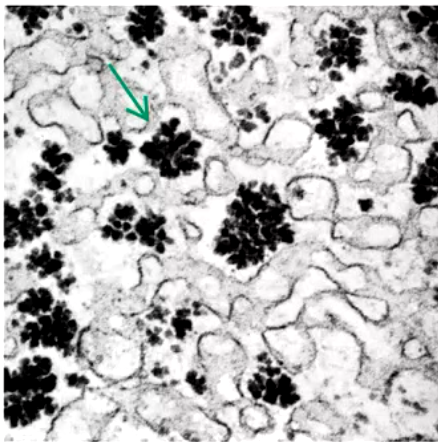
glycogen granules in a liver cell
carbo-loading (“spaghetti dinner”)
increase glycogen storage for more energy (this is limited cuz we can only store up to 500 g)
__________ is a short-term glucose buffer
glycogen
the key regulating enzyme in glycogen synthesis is __________. what does it do?
glycogen synthase
elongates an existing alpha-1,4 chain by one glucose
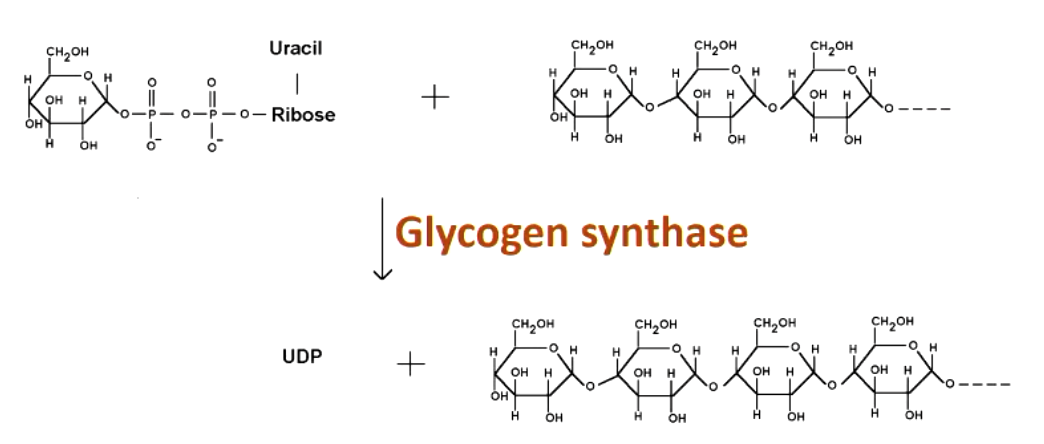
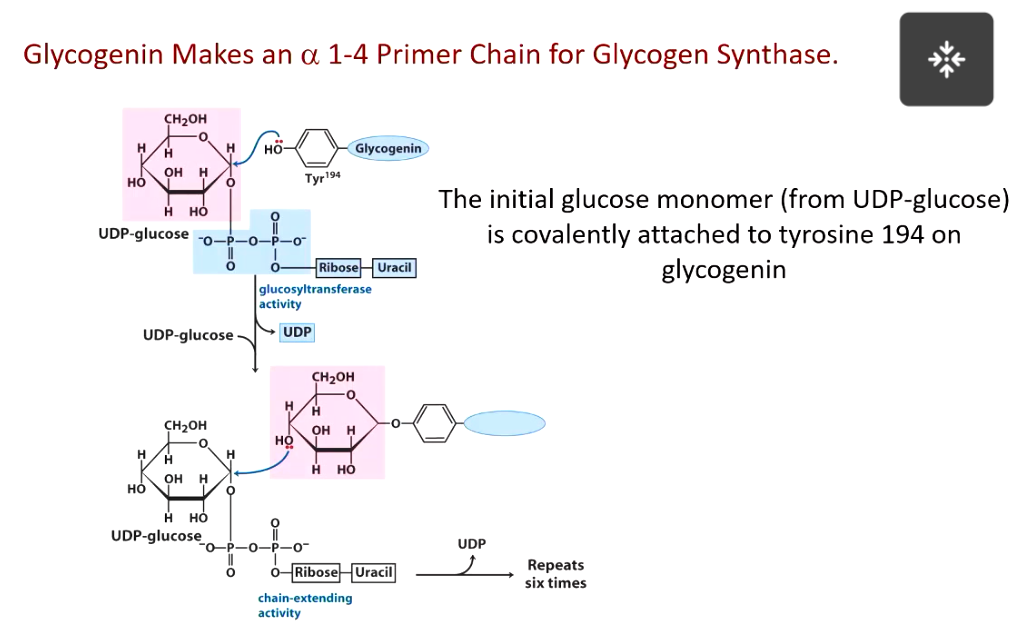
glycogen synthase requires
primer made by glycogenin
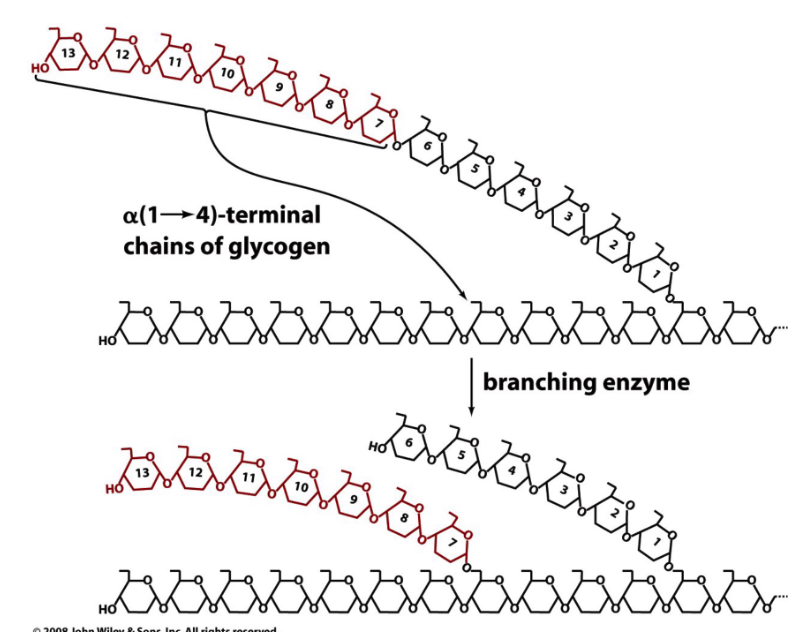
what does branching enzyme do in glycogen synthesis?
terminal 7 glucoses of a growing chain transferred to form a branch linked alpha 1-6 to a different chain (when u break it down, lots of places to start, mobilize glyocgen quickly)
2 enzymes important for glycogen synthesis
glycgoen synthase
branching enzyme
2 enzymes important for glycogen breakdown
glycogen phosphorylase
debranching enzyme
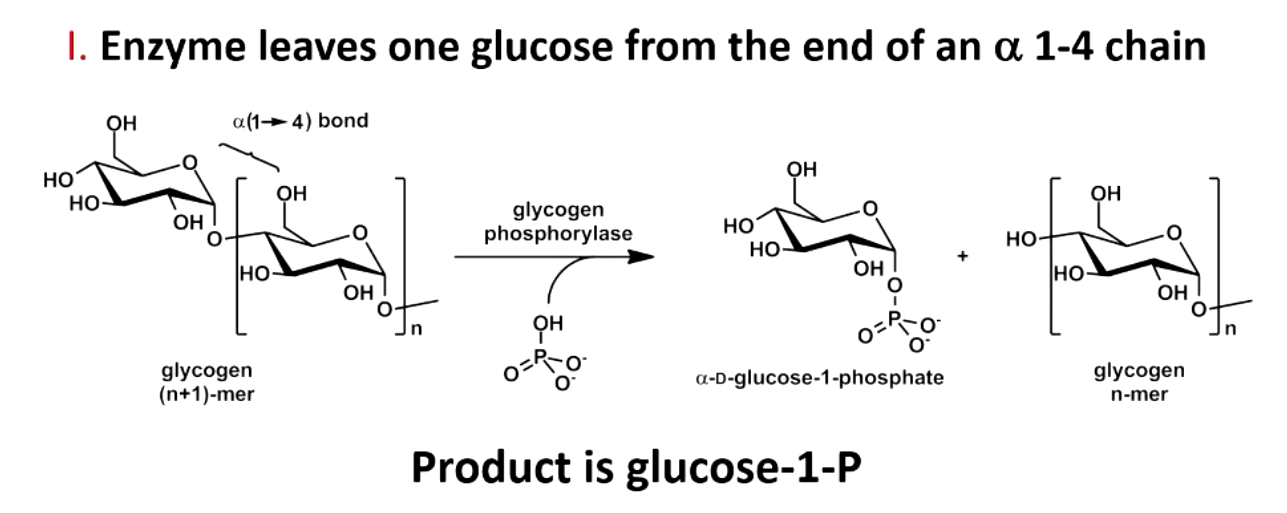
what does glycogen phosphorylase do
rate limiting, regulated step
leaves one glucose from end of alpha 1,4 chain (produces glucose 1-P)
allows very rapid generation of glucose
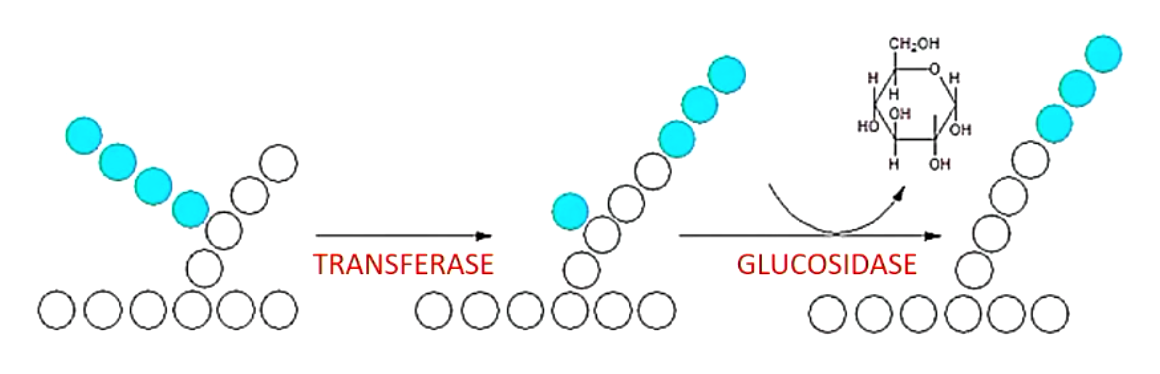
what does branching enzyme do? (2 activities)
transfers 3 residues of branching chain to 4’ end of a different chain (transferase)
cleaves remaining glucose to produce 1 free glucose (glucosidase)
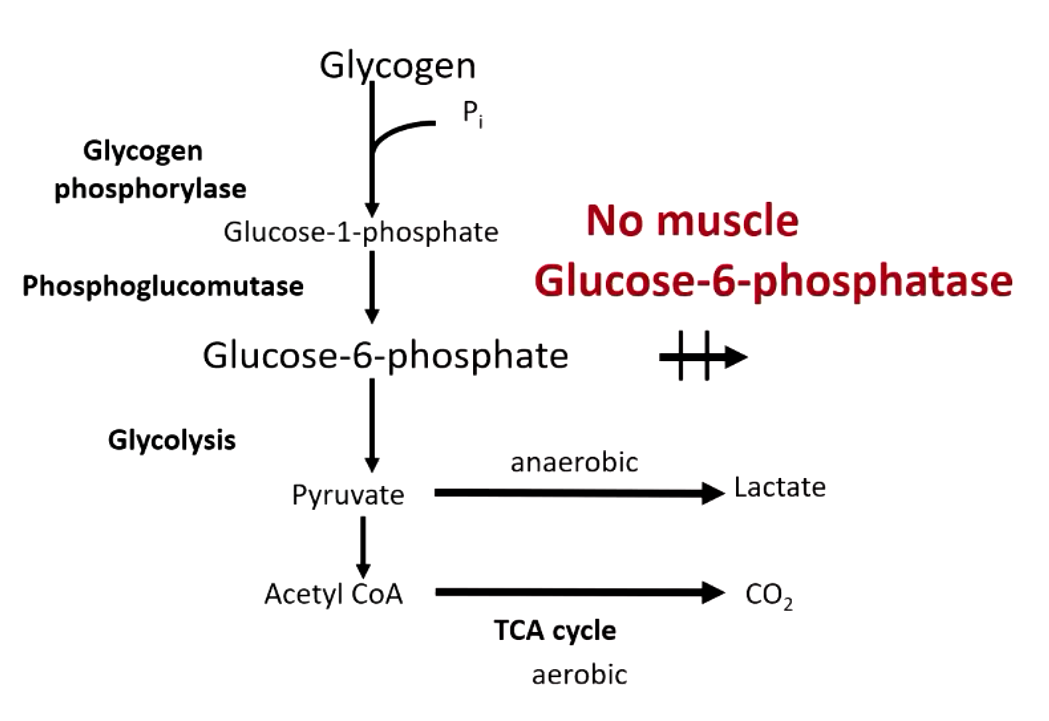
difference between glycogen breakdown in liver vs muscle
liver → doesn’t go to glycolysis, glucose produced from G6P via glucose 6 phosphatase
muscle (reserves glucose for its own use) → goes to glycolysis, no glucose 6 phosphatase in the muscle
regulators of glycogen synthesis
dietary intake (high glucose, glucose 6-P, glucose 1-P)
autoregulation (increased glycogen slows synthesis)
hormonal (insulin, glucagon, cortisol)
regulators of glycogen breakdown
AMP activates
fructose 1-P inhibits
nerve-muscle stimulus increases breakdown
glycogen phosphorylase and synthase are hormonally regulated by
glucagon and insulin
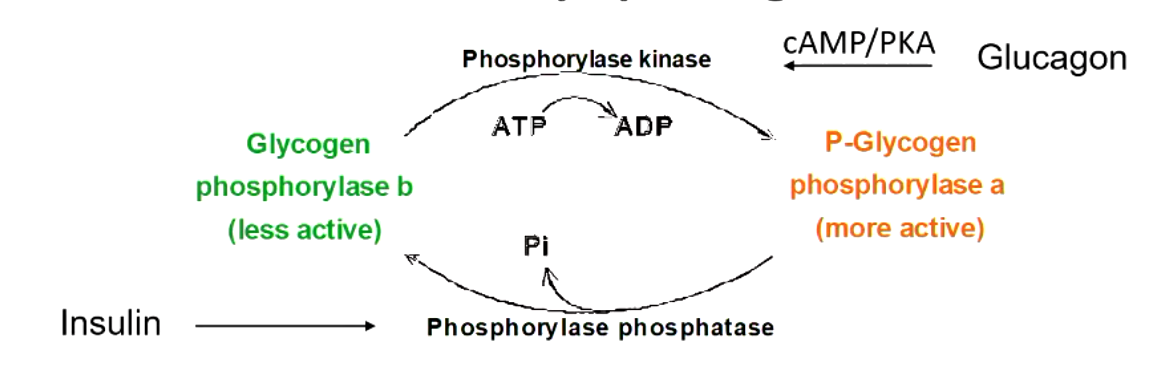
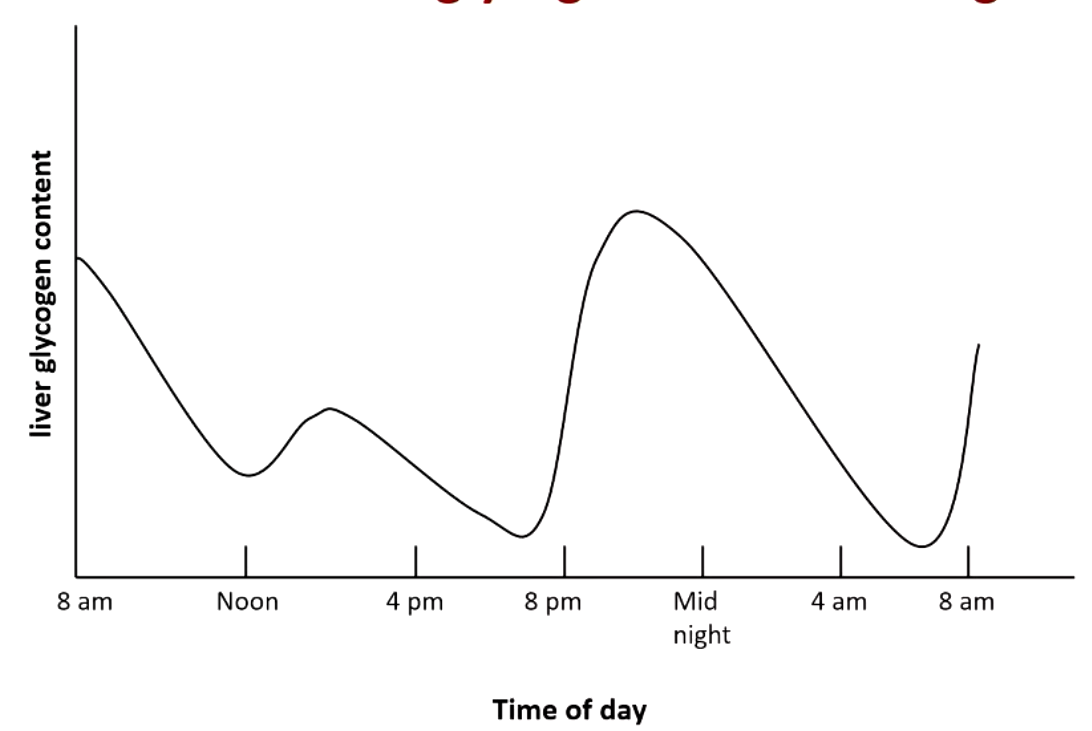
explain what this figure means
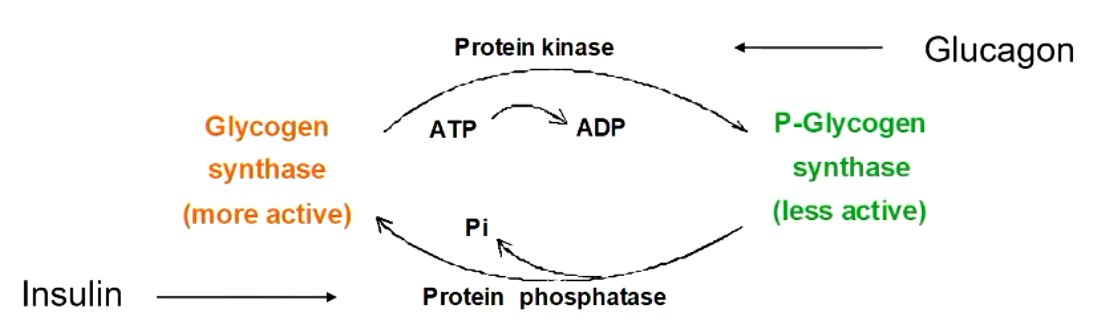
explain what this figure means
glycogen is the main form and reaches its peak at about __ hours after eating. but after ___ hours is no longer the majority and after __ hours it’s pretty much gone
glycogen is the main form and reaches its peak at about 8 hours after eating. but after 16 hours is no longer the majority and after 30 hours it’s pretty much gone
gluconeogenesis starts around __ hours after eating and becomes increasingly important. the majority form at hours and is the only form __ hours.
gluconeogenesis starts around 4 hours after eating and becomes increasingly important. the majority form at 16 hours and is the only form 30 hours.
gluconeogenesis requires
energy (4 ATP, 2GTP, 2NADH)
gluconeogenesis starts by using small molecule precursors such as…
this stpe occurs in the
lactate, alanine, pyruvate, amino acids (convertible to oxaloacetate/glycerol)
mitochondria
where does gluconeogenesis occur? (tissue)
liver (80-90%)
kidney (10-20%)
where does gluconeogenesis occur? (on a subcellular location)
mitochondria (pyruvate carboxylase)
cytoplasm
ER (glucose6-phosphatase)
the 3 regulating enzymes of glycolysis are different in gluconeogenesis
glycolysis: hexokinase, PFK, pyruvate kinase
gluconeogenesis: glucose-6-phosphatase, fructose 1,6 bisphophatase, PEP carboxykinase/pyurvate carboxylate
how does oxaloacetate inside the mitochondria come out into the cytoplams during gluconeogenesis?
oxaloacetate is converted to aspartate or (more commonly) malate which can cross mitochondrial membrane
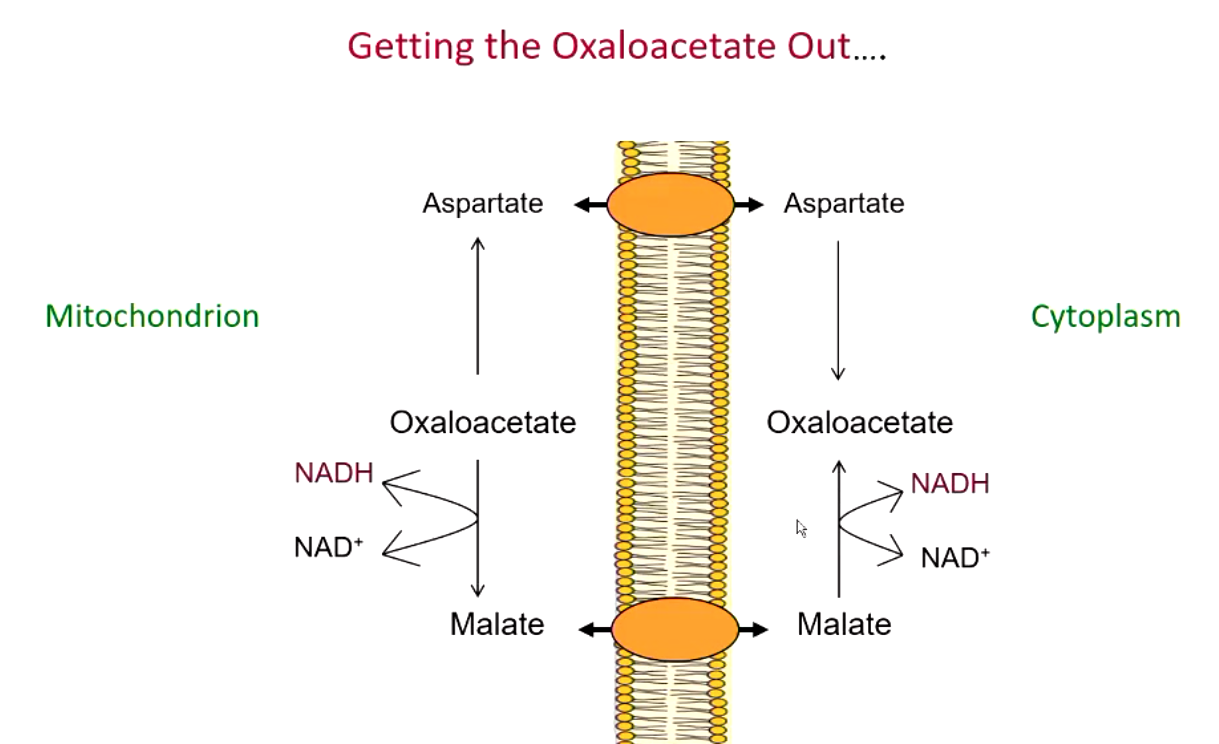
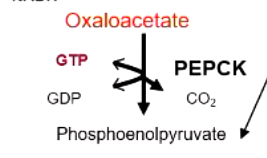
major regulating step in gluconeogenesis
PEP carboxykinase
glycogen storage disease type I
glucose-6-phosphatase defect (von gierke disease)
hypoglycemia occurs from inability of liver to generate glucose either by glycogenolysis or gluconeogenesis
type 1A is a glucose 6-phosphatase defect
type 1B transporter defect (glucose 6 phosphate cant get transported to be hydrolyzed) has important dental consequences
glycogen storage disease type II
lysosomal alpha 1-4 glucosidase defect (pompe disease)