Base Properties of Amines
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Ranking the base strength of amines
Aliphatic amines are stronger bases than ammonia, which is a stronger base than aromatic amines.
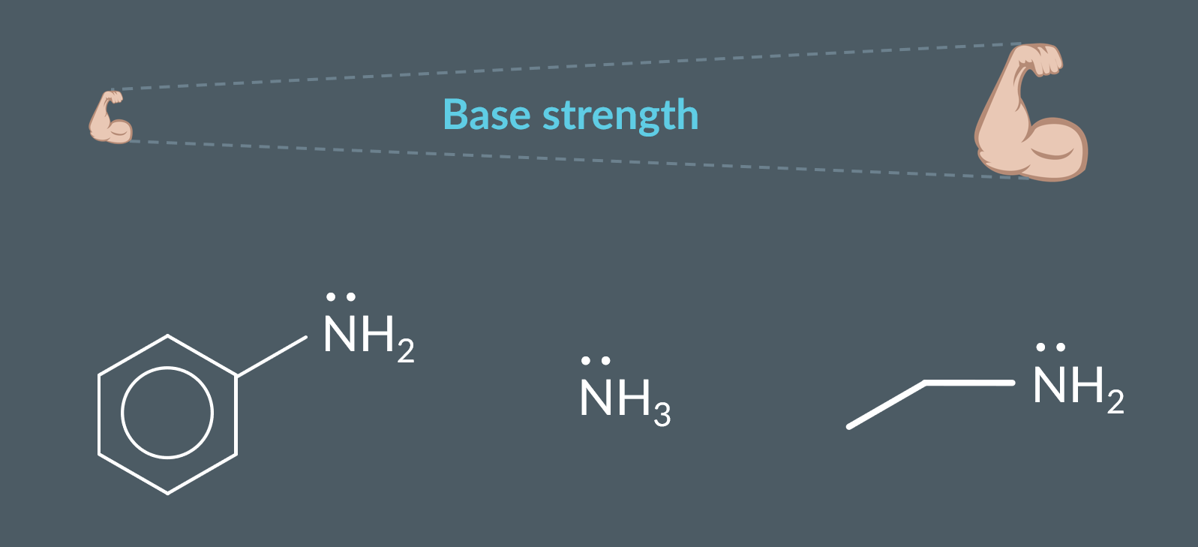
what is amine’s base strength is determined by
by how available the lone pair on its nitrogen is.
how does availiailty affect the stronger the base.
The more available its lone pair is to accept a proton, the stronger the base.
why is aliphatic amine stronger base
In an aliphatic amine, the alkyl groups donate electron density to the nitrogen. This means the lone pair is better at accepting a proton, so it’s more available than the lone pair in ammonia.
why is aromatic amine weaker base
an aromatic amine, the lone pair gets delocalised into the pi system. This makes the lone pair less available than ammonia’s.
Base strength of amines with multiple alkylgroups
The more alkyl groups there are, the more available nitrogen’s lone pair will be, so the base will be stronger.
Base strength of amines with multiple aromatic groups
the more benzene rings there are bonded to the nitrogen, the less available the lone pair will be, so the base will be weaker.