Science Midterm Review Flashcards
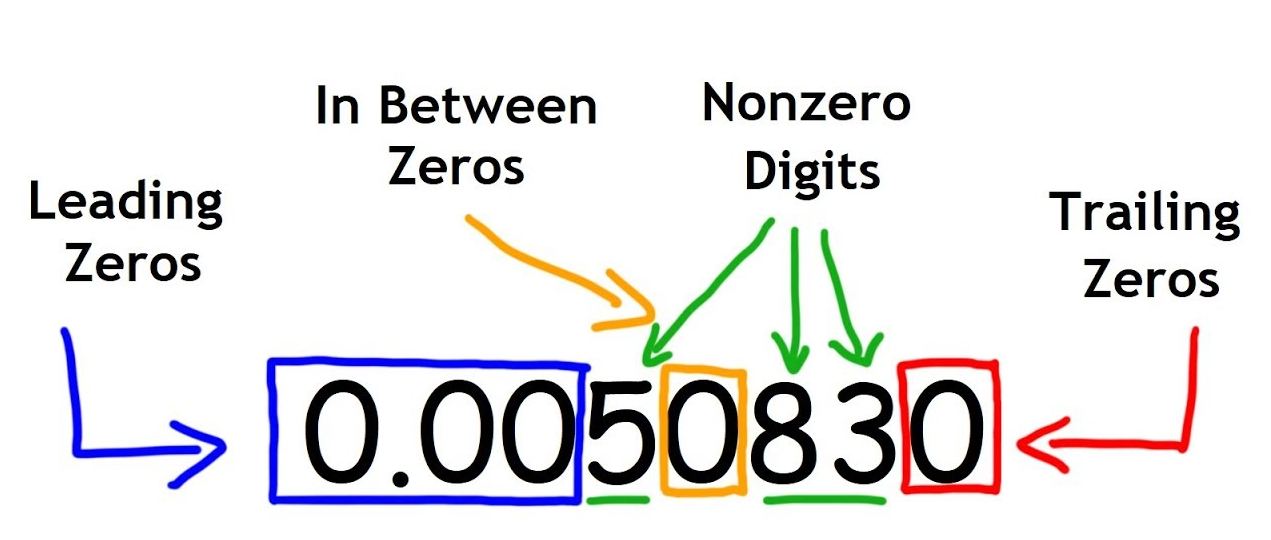
Significant Figures
all non-zero digits
any zeros that are contained between non-zero digits
leading zeros don’t count
trailing zeros may not count if there is no decimal point
Scientific Notation
shorter way to write a long number
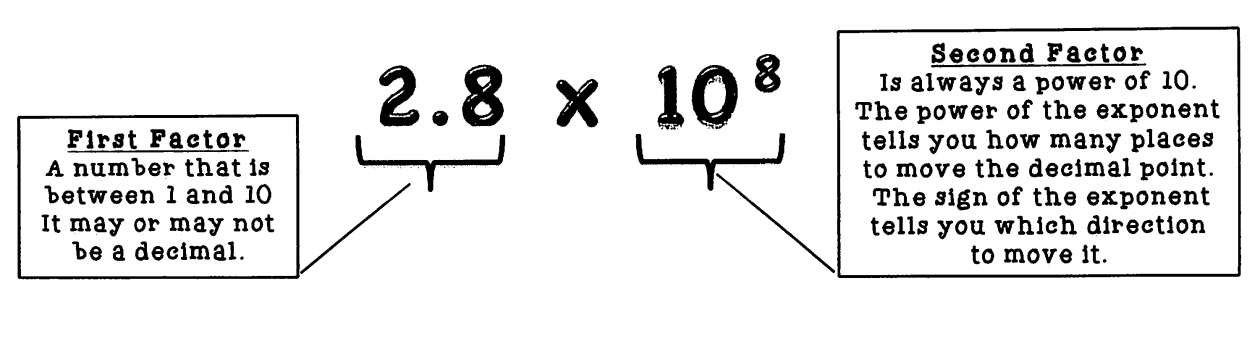
Steps:
put the decimal after the first significant figure
indicate how many places the decimal moved by the power of 10
positive = decimal moved to the left
negative = decimal moved to the right
The Basics of Matter
Vocab
matter - anything that has mass + takes up space
atom - smallest particle of an element
Element - a substance composed of atoms having an identical number of protons in each nucleus. Elements cannot be reduced to simpler substances by normal chemical means.
Compound - a pure, homogeneous substance consisting of atoms or ions of two or more different elements in definite proportions that cannot be separated by physical means. A compound usually has properties unlike those of its constituent elements.
Mixture - a composition of two or more substances that are not chemically combined with each other and are capable being separated.
Pure substance - a sample of matter, either an element or a compound, that consists of only one component with definite physical and chemical properties and a definite composition.
Chemical change - a process where bonds are broken and new bonds are formed between different atoms.
Physical change - a usually reversible change in the physical properties of a substance, as size or shape.
Gas – a substance having no definite shape and no definite volume. The molecules are spread out and free to move.
Liquid – a substance having a definite volume, but no definite shape. The inter-molecular forces hold these atoms or molecules loosely together but do not force them into a rigid structure allowing liquids the ability to flow.
Solid - a substance having a definite shape and definite volume. The atoms or molecules are held in rigid structure. Although they are free to vibrate, they cannot move around
Plasma - gases that have been so energized that their atoms have been stripped of some or all electrons
Pressure - the force exerted on a surface per unit area
Volume - the amount of space that a substance or object occupies

Mixtures
a mix of elements and compounds NOT chemically bonded
can be Heterogeneous or Homogeneous
heterogeneous is when the arrangement of particles in not uniform
homogeneous is when the arrangement of particles is uniform
Pure Substances
can be elements
singular atoms
can be compounds
chemically bonded elements
Volume
cube/rectangular prism: l x w x h
use the Water Displacement method for irregularly shaped objects
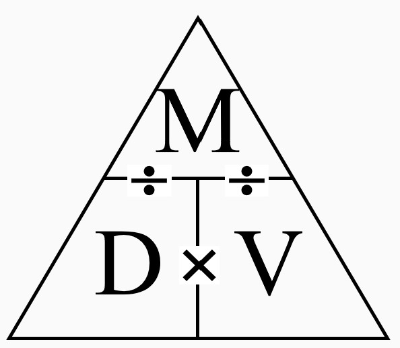
Density
mass/volume
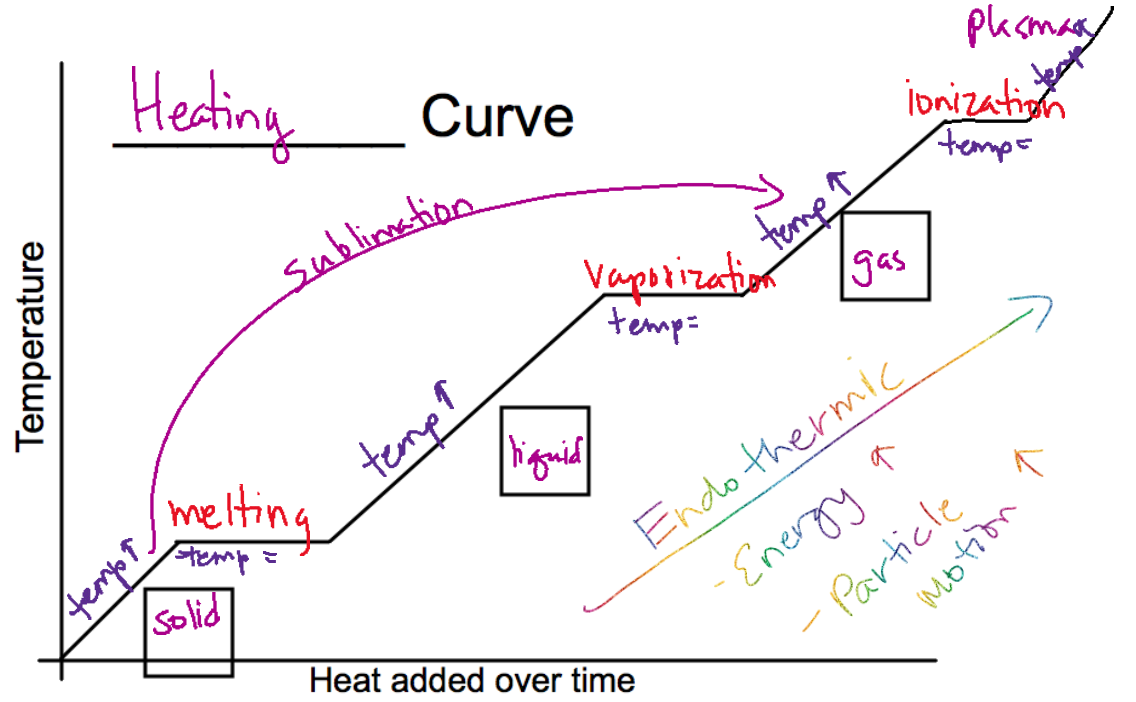
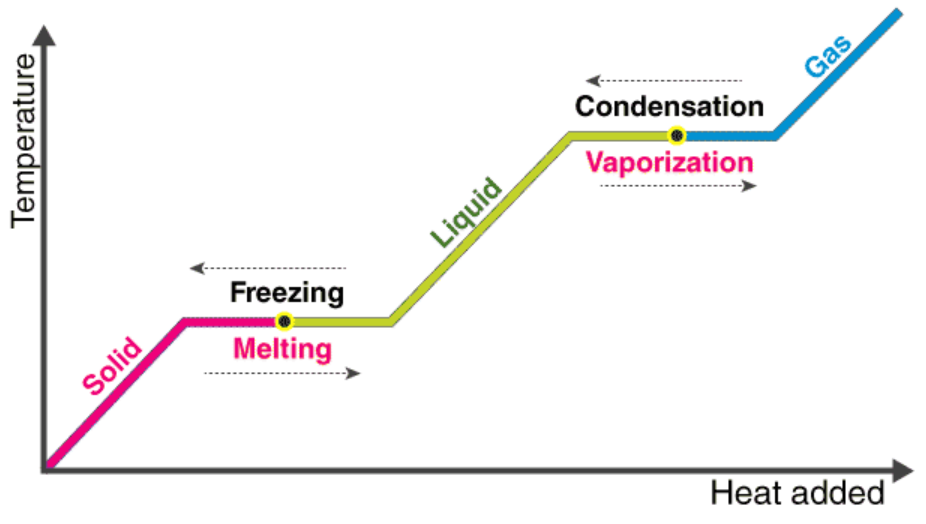
Phase Changes + Heat
Phase Changes
Melting - solid to liquid
Freezing - liquid to solid
Vaporization - liquid to gas
Condensation - gas to liquid
Sublimation - solid to gas
Deposition - gas to solid

kinetic energy: the energy of an object because of motion
temperature measures the average kinetic energy of the particles of on object or substance
measured in degrees Celsius or Kelvins
heat is the flow of energy from warmer places to cooler places due to a difference in temperature
thermal equilibrium is when heat flows from a higher temperature object to a lower temperature object, until they are the same temperature
heat can transfer in 3 different ways
Conduction: the transfer of heat energy between materials that are in direct contact with each other.
ex: the handle of a metal spoon that has been placed in a bowl of hot soup. The hot soup transfers heat to the end of the spoon; the heat is then transferred through the spoon to the handle.
Convection: the transfer of heat energy by the mass movement of fluids containing heated particles
convection currents
when particles of a fluid are heated, the particles move farther apart, causing the fluid to expand
ex: home heating systems force heated air into rooms by way of convection currents; these currents heat the colder air in the room.
Radiation: the transfer of heat energy through electromagnetic waves.
electromagnetic waves originate from accelerated charged particles
electromagnetic waves travel through matter or through empty space
heat transfer through empty space is unique to radiation.
ex: since the space between the Sun and Earth is essentially a vacuum, the heat energy from the Sun is transferred to Earth only by radiation.
endothermic: energy going into the system
exothermic: energy going out of the system
specific heat is the energy needed to raise substance temperature
varies for each substance
measured in joules per gram per degree Celsius (J/g°C) or calories per gram per degree Celsius (cal/g°C)
The equation Q = mcΔT can be used to calculate heat gained or lost, where Q is heat energy, m is mass, c is specific heat, and ΔT is temperature change.
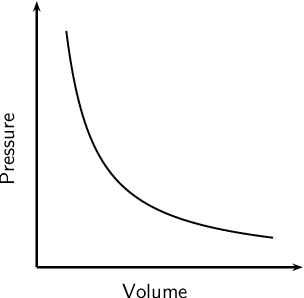
Boyle’s Law
the volume of a given mass of gas varies inversely with the pressure when the temperature is kept constant
We use P1 and V1 to stand for the initial pressure and initial volume of a gas. After a change has been made, P2 and V2 stand for the final pressure and volume. The mathematical relationship of Boyle's Law becomes:
P 1 × V 1 = P 2 × V 2
2 changing variables - pressure + volume
constant variable - temperature
inverse relationship
real life example: syringe with marshmallow inside
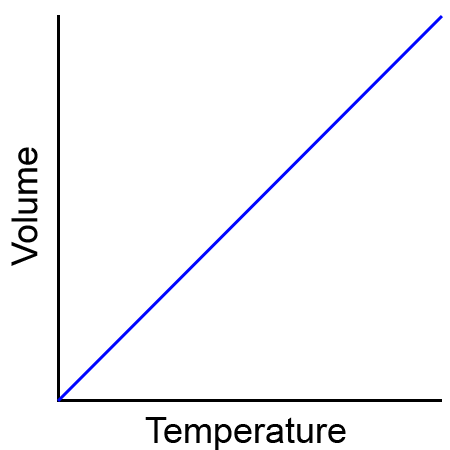
Charles’s Law
the volume of a given mass of gas varies directly with the temperature of the gas when the pressure is kept constant
We will use V1 and T1 to stand for the initial volume and temperature of a gas, while V2 and T2 stand for the final volume and temperature. The mathematical relationship of Charles's Law becomes:

2 changing variables - volume + temperature
constant variable - pressure
direct relationship
real life example: tire cold in the winter (temperature decreases) ➡ pressure decreases
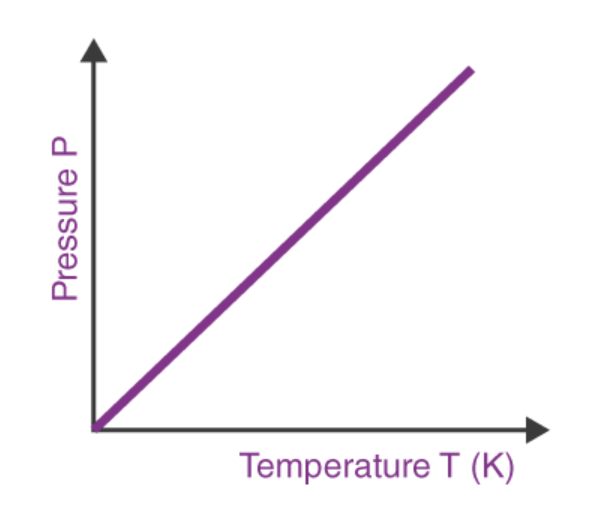
Gay-Lussac’s Law
the temperature of gas varies directly with the pressure when the volume is kept constant
We will use V1 , P1, and T1 to stand for the initial volume, pressure, and temperature of a gas, while V2, P2, and T2 stand for the final volume, pressure, and temperature. The mathematical relationship of Charles's Law becomes:
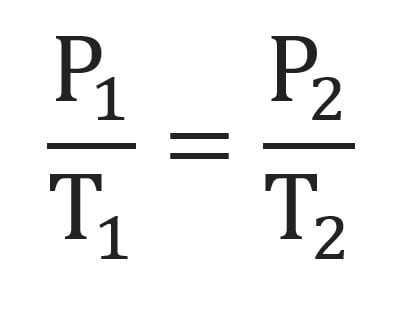
2 changing variables - pressure + temperature
constant variable - volume
direct relationship
real life example: rice cooker gets heated up (temperature increases), and pressure increases inside of the cooker; this causes the whistle to blow
Atoms
Bohr Model
electrons
negative charge
makes up the least amount of mass in an atom
orbit the nucleus
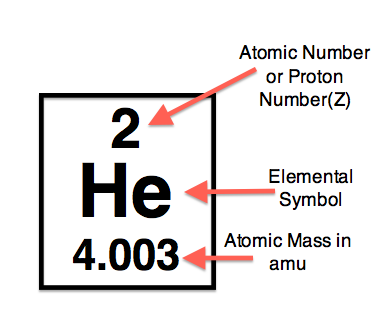
protons
positive charge
mass = 1 amu
inside nucleus
neutrons
neutral/no charge
mass = 1 amu
inside nucleus
● Isotopes - atoms with different numbers of neutrons
● Ions - atoms with a charge
● Average atomic mass - the number of protons + neutrons
● Mass number - the average atomic mass rounded to the nearest
whole number
Periodic Table
18 groups
7 periods
Same group = same properties + # of valance electrons
Same period = same number of shells
the one’s place in a group’s number tells the # of valance electrons
metals on right
metalloids in middle
non-metals on right
alkali metals
highly reactive
group 1
alkaline earth metals
quite reactive
group 2
halogen family
most reactive non-metals
group 17
noble gases
stable
group 18
Octet Rule - the max number of valence electrons any element can have is 8 (stable)
Lewis Dot Diagrams
the amount of valance electrons in an atom represented with dots

Chemical Bonding
ionic bonding
metal and nonmetal
gives/takes valance electron(s)
swap oxidation numbers to find the bond
can conduct electricity
use Lewis Dot Diagrams
covalent bonding
nonmetal (x2)
shares valance electron(s)
use Lewis Dot Diagrams
ionic naming
name of metal
name of non-metal + “ide”
subscript DOESN’T change the name
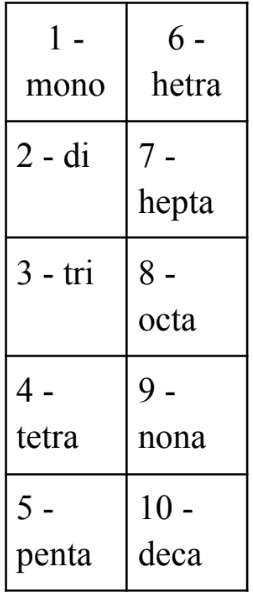
covalent naming
1st non-metal name + prefix (subscript)
2nd non-metal name + “ide”
Law of Conservation of Mass - Mass can neither be created nor destroyed; it can only be changed from one form to another.
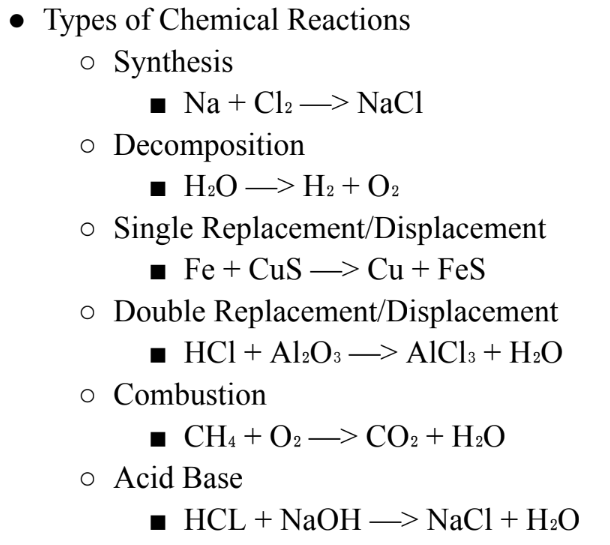
Solutions + Acids + Bases
Solute - smaller part that gets dissolved
Solvent - larger part that dissolves the solvent
Water = universal solvent
Solute + solvent = solution
Solubility - grams of solute that can be dissolved into 100 grams of H2O at a given temperature
Saturated Solution - cannot take anymore solution; will settle at the bottom
Unsaturated Solution - can hold more solution
Super-saturated Solution - holding more solute than the solution can actually hold
Dilute - very little solute in solvent
Strong - a lot of solute in solvent
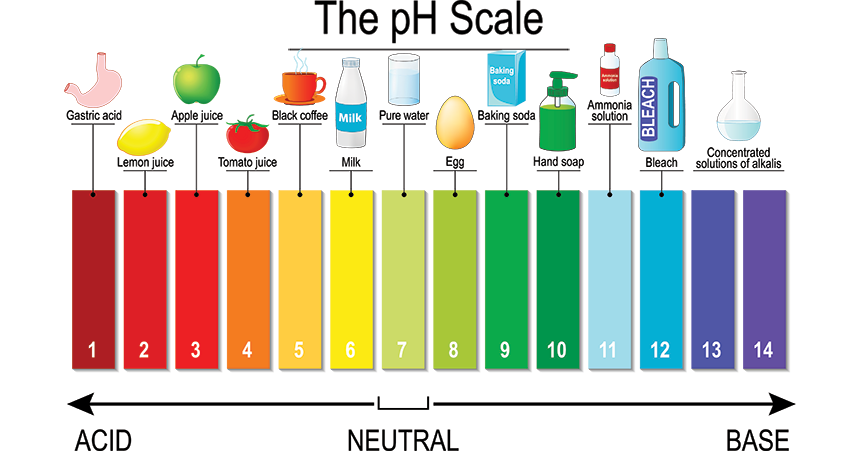
Acids
Hurt skin
Can corrode metals
Sour to taste
Turns blue litmus paper to red
Acids turn red cabbage juice pink
When submerged in water, acids release the H+ ion
Bases
Slippery to touch
Bitter to taste
Turns red litmus paper to blue
Bases turn red cabbage juice green
When submerged in water, bases release the OH- ion