gases chem unit 4
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Down
Pressure and volume are inversely related meaning if pressure goes up volume goes _____
Directly
Volume and temperature are _____ proportional so if volume decreases, temperature decreases too
Down
Pressure and temperature are directly proportional meaning if pressure goes down, temperature goes ____
Higher speed/velocity and lighter molar mass
Lower curve towards the right indicates
Slower speed and heavier molar mass
Higher curves shifted left indicate
Increases
As molar mass decreases, average speed
Increases
As temperature increases, average speed of particles
Lower
Higher curves shifted left indicate a ____ temperature
Higher
Lower curves shifted right indicate a ____ temperature
It acts more differently from an ideal gas-greater intermolecular forces than an ideal gas
If something deviates more from an ideal gas that means
Lead to a lower measured pressure than the ideal gas law predicts
A gas that is NOT an ideal gas/intermolecular forces dominate will
Measured pressure will be closer to what the ideal gas law predicts
If high temperature dominates,
High, low
Ideal gases behave most ideally at ____ temperatures and _____ pressures
Low, high
Element gases behave LEAST like an ideal gas(deviation) at _____ temperatures and ____ pressures
No effect it stays the same
Volume is changed from 4L to 1L and temperature is held constant. What effect does the volume and pressure have the average kinetic energy?
5L of CO2 and 10L of H2O
CH4 (g) + 2O2,(g) → CO2(g) + 2H20(g)
If you start with 10.0 Lof O2, gas, how many liters of CO, and H,0 gas will be produced, assuming the reaction goes to completion and all gases are measured at the same temperature and pressure?
30L
N₂(g) + 3H₂(g) → 2NH₃(g)
If you begin with 15.0 liters of N₂ gas, how many liters of NH₃ gas will be produced, assuming the reaction proceeds to completion and all gases are measured under the same conditions of temperature and pressure
8L
2CO(g) + O₂(g) → 2CO₂(g)
If you start with 8 liters of CO and excess O₂, how many liters of CO₂ will be produced, assuming the reaction goes to completion and all gases are measured under the same conditions?
7L
2CO(g) + O₂(g) → 2CO₂(g)
If you have 7 liters of CO, how many liters of CO₂ will be produced if the reaction goes to completion?
Pressure will increase because more molecules means more can hit the wall
If you have a gas in a container (rigid), and then you add the same mass of another gas to the same container. What happens to the pressure if the temperature is constant?
6.00atm
A mixture of 12.04 x 10²³ molecules of CH₄(g) and 4.00 x 10²³ molecules of He(g) has a total pressure of 9.00 atm. What is the partial pressure of CH₄(g)?
(A) 1.00 atm
(B) 2.00 atm
(C) 6.00 atm
(D) 8.00 atm
Container 4
Four samples of gases are at 300 K in identical rigid metal containers under the conditions given in the table below.
| Container | Gas | Pressure (atm) | Mass of sample (g) |
|---|---|---|---|
| 1 | CH₄ | 2.00 | 8 |
| 2 | N₂ | 2.00 | 28 |
| 3 | O₂ | 2.00 | 32 |
| 4 | He | 2.00 | 2 |
Under the conditions given, the average speed of the gas particles is:
(A) greatest in container 1
(B) greatest in container 2
(C) greatest in container 3
(D) greatest in container 4
Faster
According to effusion, the lighter the gas the ____ it will leak out of the container
Slower
According to effusion, the heavier the gas the ____ it will leak out of the container
Faster they will mix
According to diffusion, the lighter an acid or base weighs, the _____
HCl and KOH
A common chemistry demonstration involves observing the diffusion of gases in a glass tube. Cotton swabs soaked in an acid and a base are placed at opposite ends of the tube. As the gases volatilize and meet, they react to form a solid precipitate. Which combination of acid and base will result in the solid forming closest to the BASE?
(A) HBr and KOH
(B) HCl and KOH
(C) HBr and LiOH
(D) HCl and LiOH
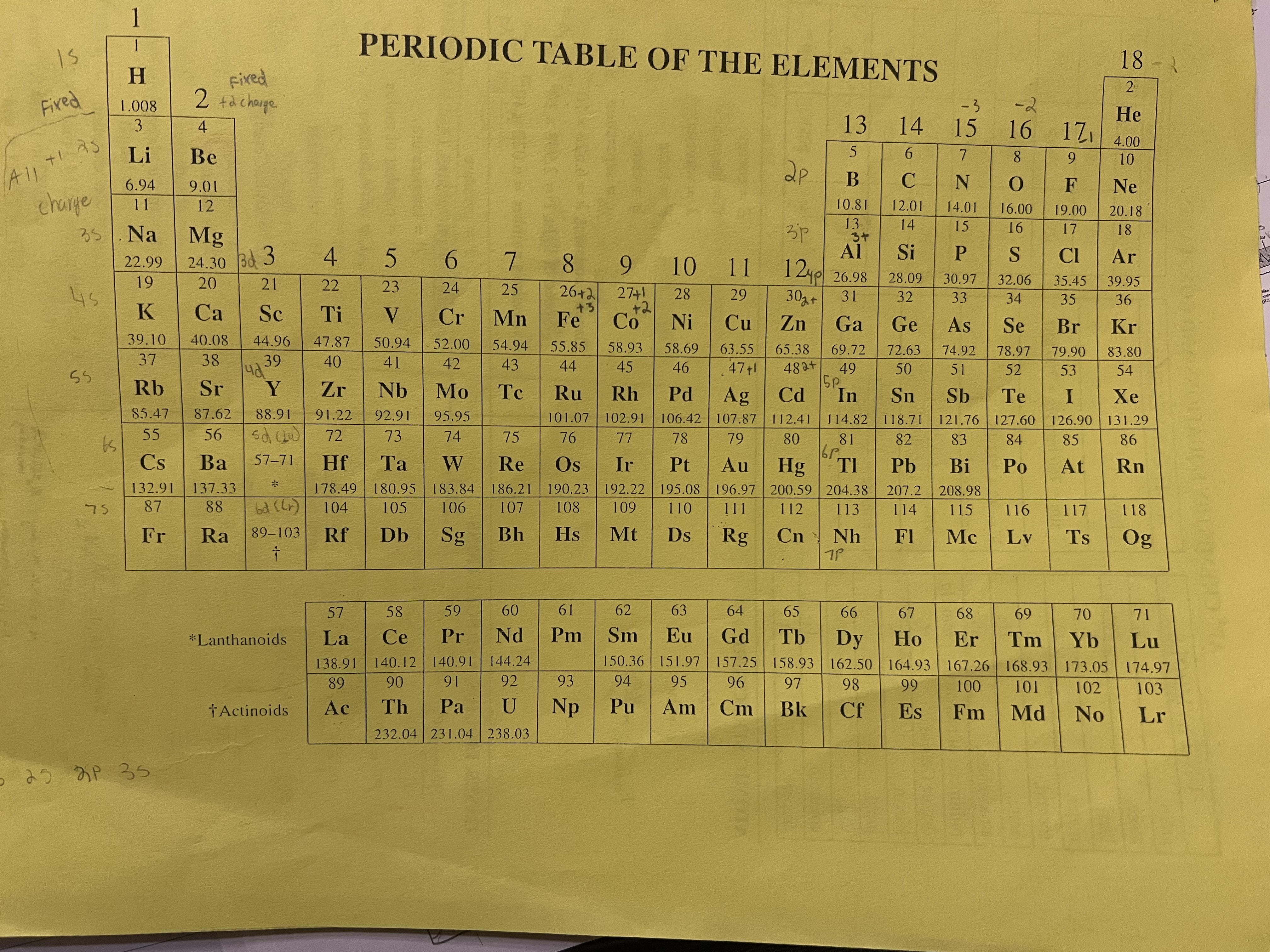
Slowest
Whenever you get a question on acid and base, whatever one they want it closest to is gonna be the one that you want to be the _____
HBr and NaOH
Which combination of acid and base will result in the solid forming closest to the ACID?
* (A) HF and RbOH
* (B) HBr and NaOH
* (C) HF and NaOH
* (D) HBr and RbOH
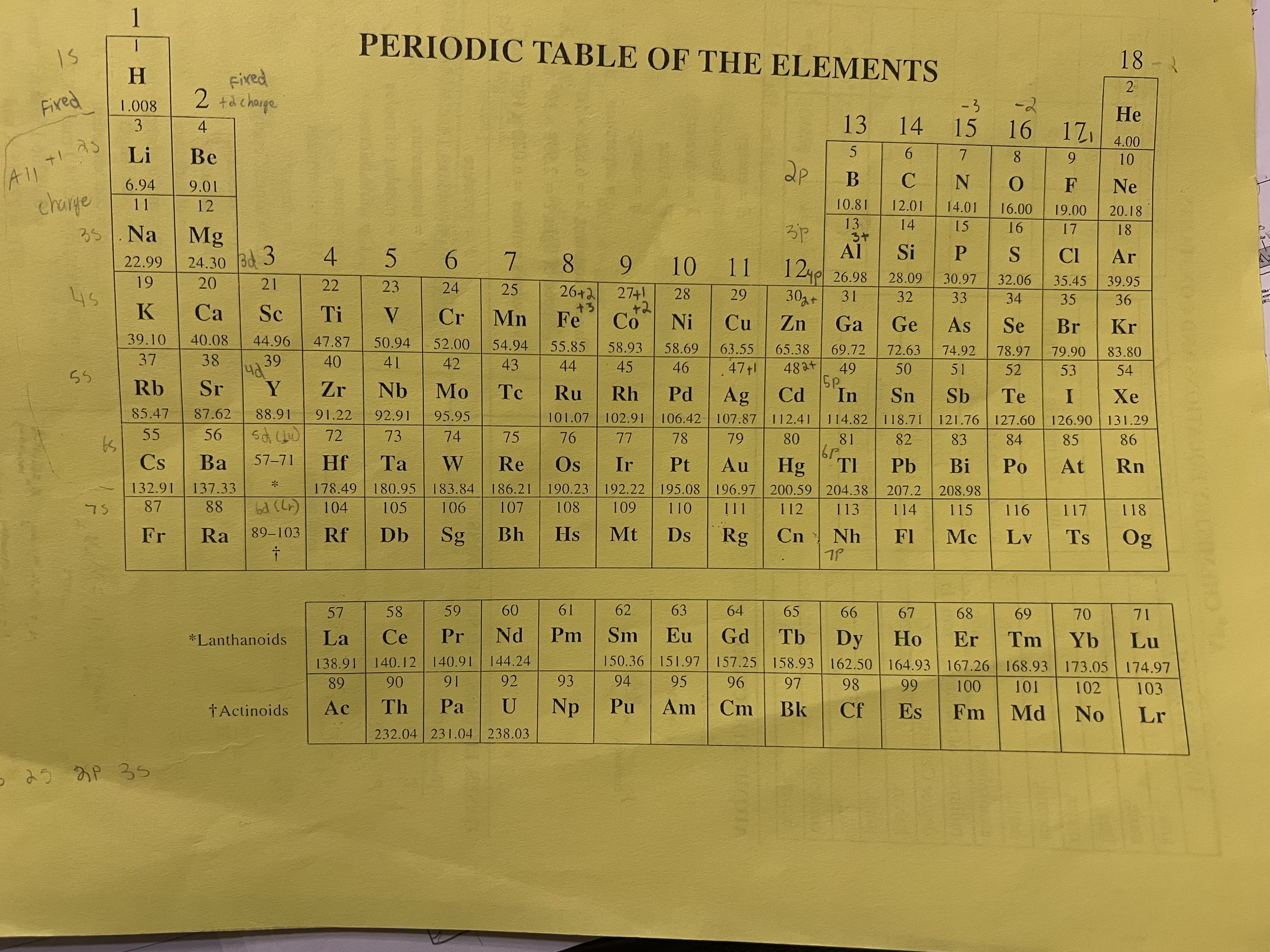
M=m/n
(molar mass) formula
As temperature lowers, the avg kinetic energy of the PARTICLES lowers, meaning they will be hitting the sides of the container with less force/not as often.
Why would lowering the temperature of a gas cause the pressure to decrease in the container?