Chemistry
5.0(1)
5.0(1)
Card Sorting
1/93
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
94 Terms
1
New cards
define element
A substance that cannot be broken down into simpler substances by chemical means.
2
New cards
define compound
a substance made up of two or more different chemical elements combined in a fixed ratio
3
New cards
define mixture
a physical combination of two or more substances that aren't chemically joined
4
New cards
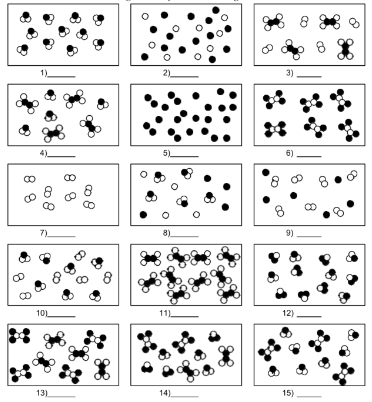
What is number 1?
pure substance of compound molecules
5
New cards
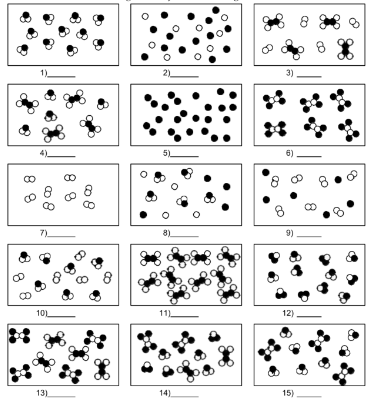
What is number 2?
mixture of element atoms
6
New cards
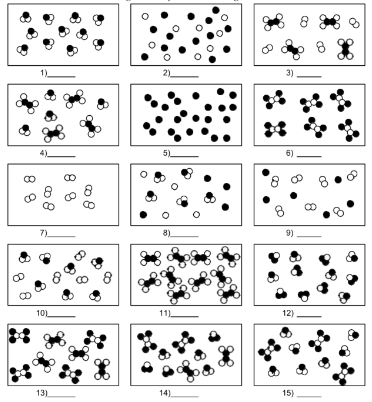
What is number 3?
mixture of element and compound molecules
7
New cards
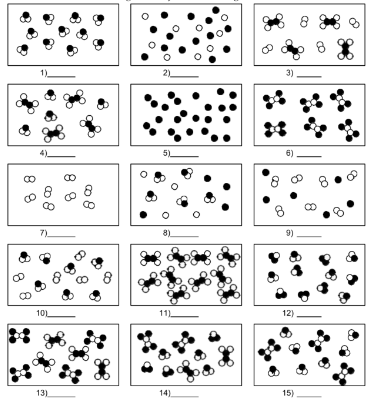
What is number 4?
mixture of compound molecules
8
New cards
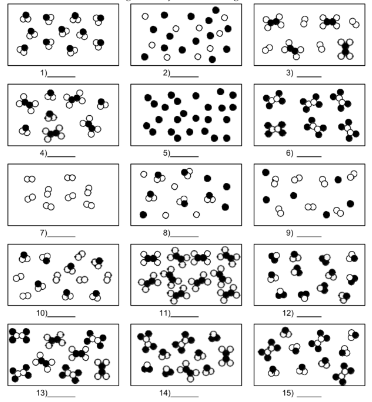
What is number 5?
pure substance of element atoms
9
New cards
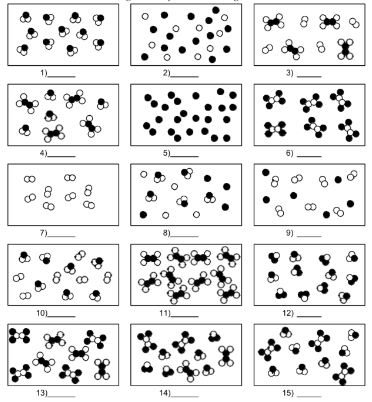
What is number 6?
pure substance of compound molecules
10
New cards
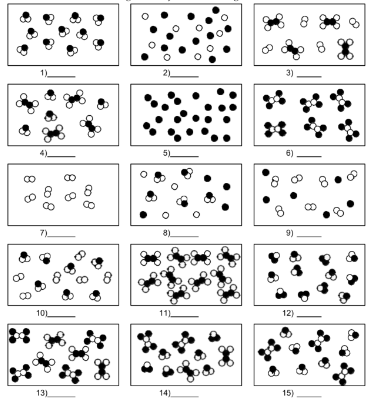
What is number 7?
pure substance of element molecules
11
New cards
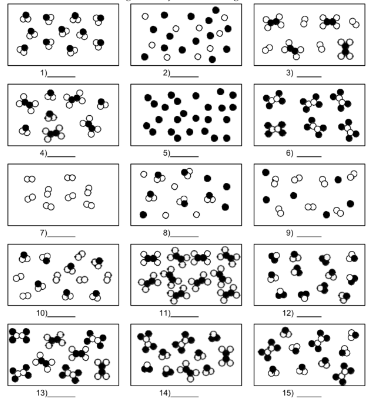
What is number 8?
mixture of element atoms and compound molecules
12
New cards
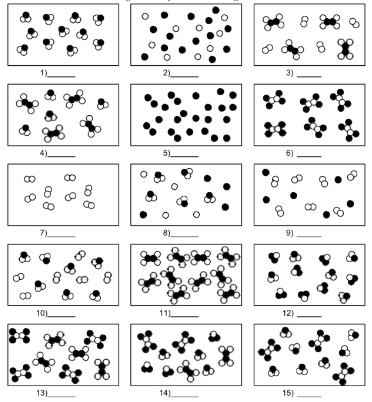
What is number 9?
mixture of element molecules and atoms
13
New cards
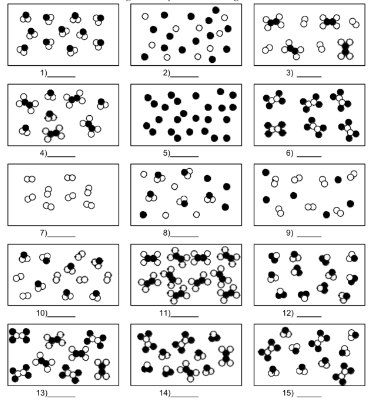
What is number 10?
mixture of element and compound molecules
14
New cards
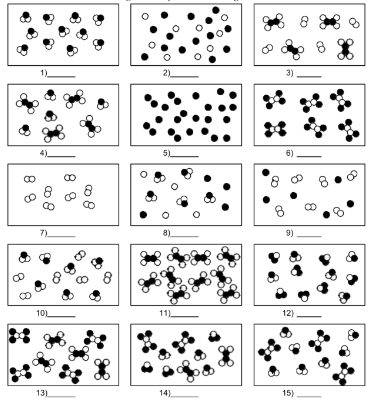
What is number 11?
pure substance of compound molecules
15
New cards
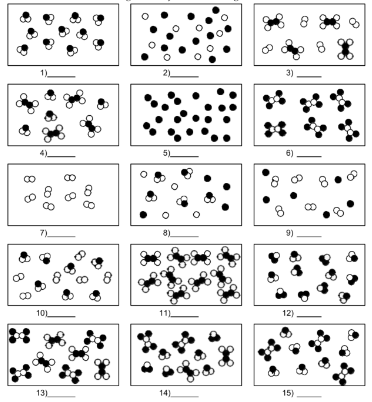
What is number 12?
mixture of compound molecules
16
New cards
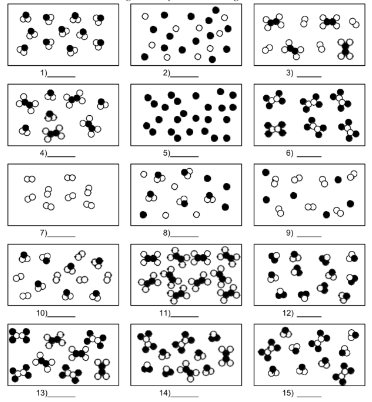
What is number 13?
mixture of compound molecules
17
New cards
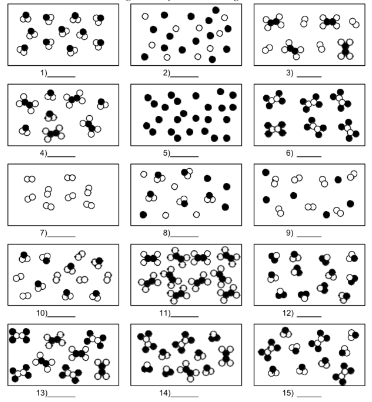
What is number 14?
mixture of compound molecules
18
New cards
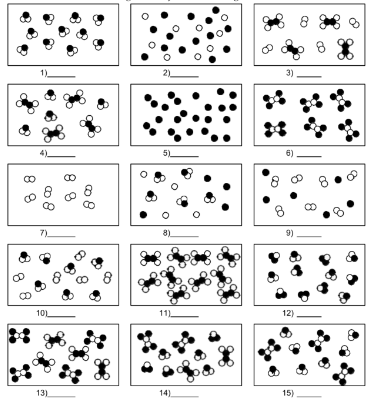
What is number 15?
mixture of compound molecules
19
New cards
how can you classify something as an element?
It contains only one type of atom
20
New cards
how do you classify something as a compound?
It contains two or more different atoms joined together in a fixed ratio
21
New cards
how do you classify something as a mixture?
It contains two or more different substances that are only physically joined together, not chemically
22
New cards
Pure substances always consist of . That matter can be an or a .
one type of matter, element, compound
23
New cards
Is oxygen an element, compound or mixture?
element
24
New cards
Is carbon dioxide an element, compound or mixture?
Compound
25
New cards
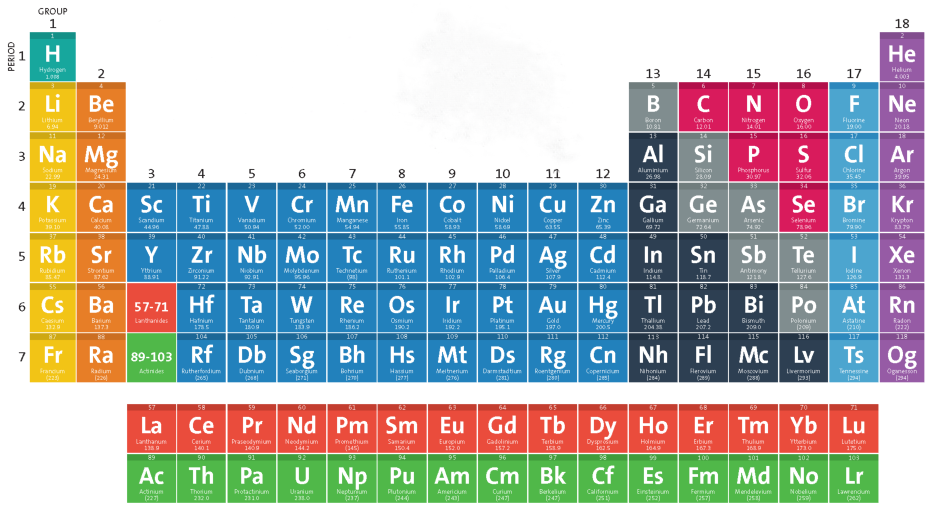
What do the different colours of the periodic table represent?
elements with similar properties or chemical reactivity
26
New cards
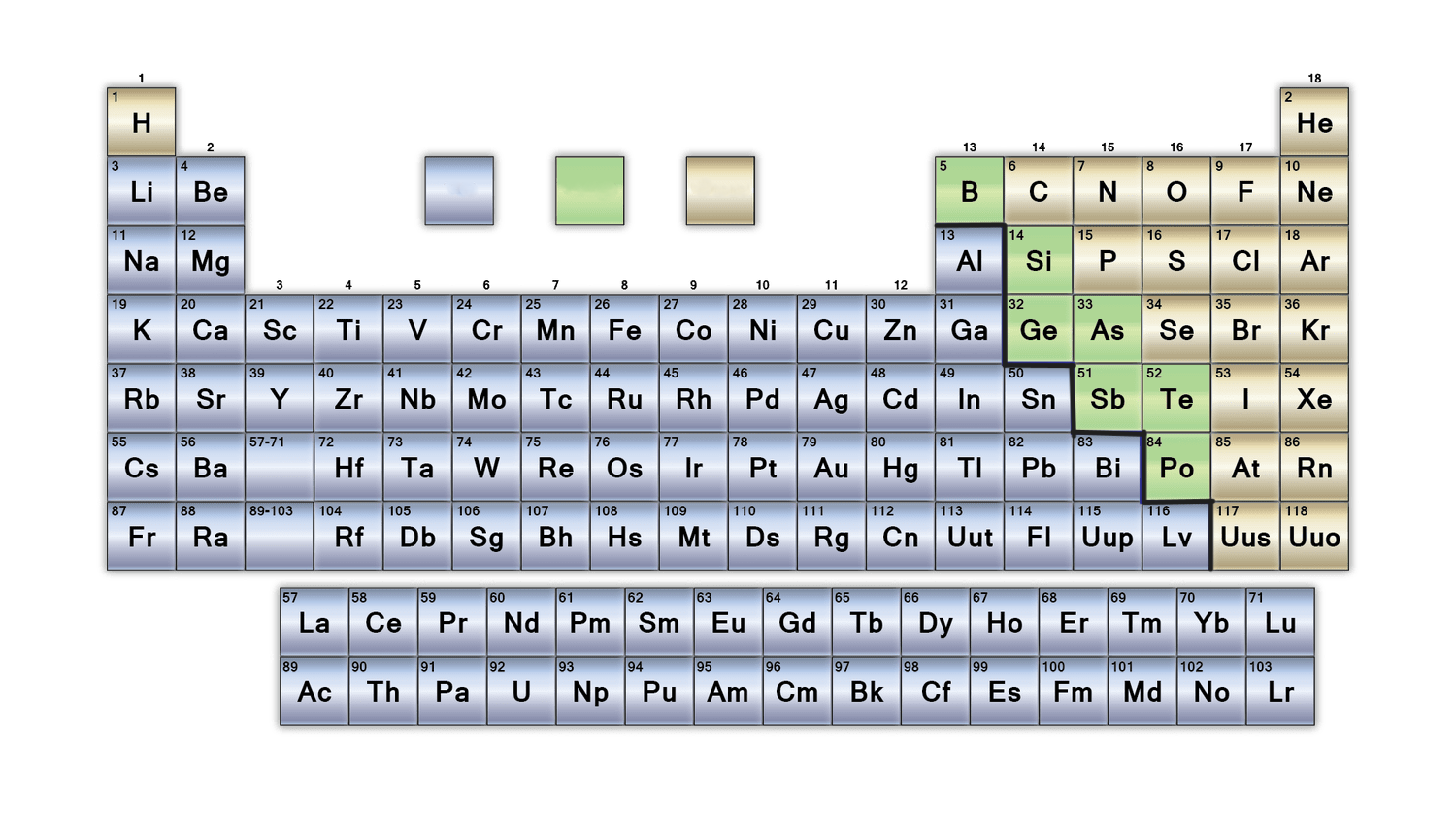
what group does the yellow colour represent in this table?
nonmetals
27
New cards
what group does the blue colour represent in this table?
metals
28
New cards
What group does the green colour represent in this table?
metalloids
29
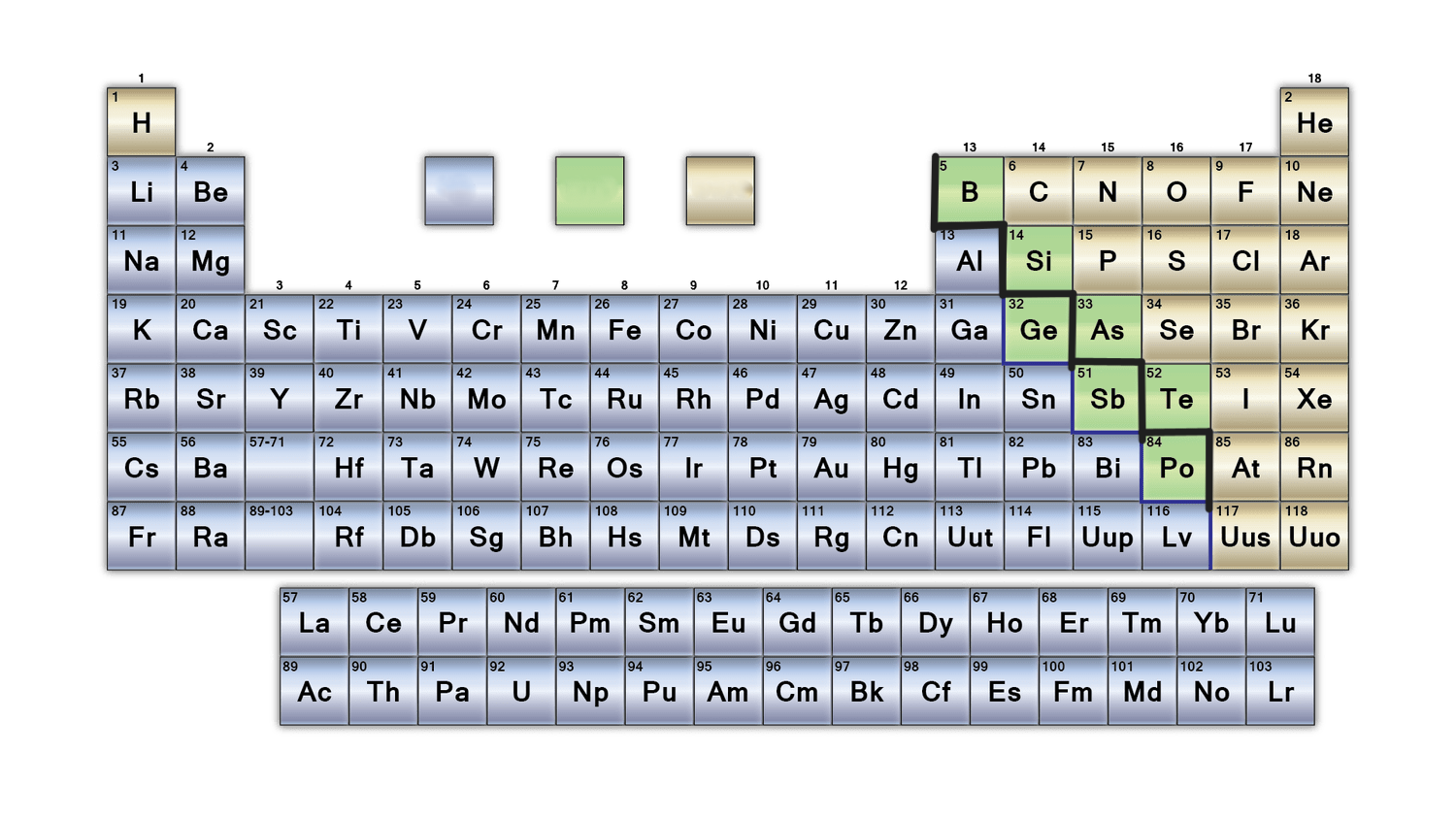
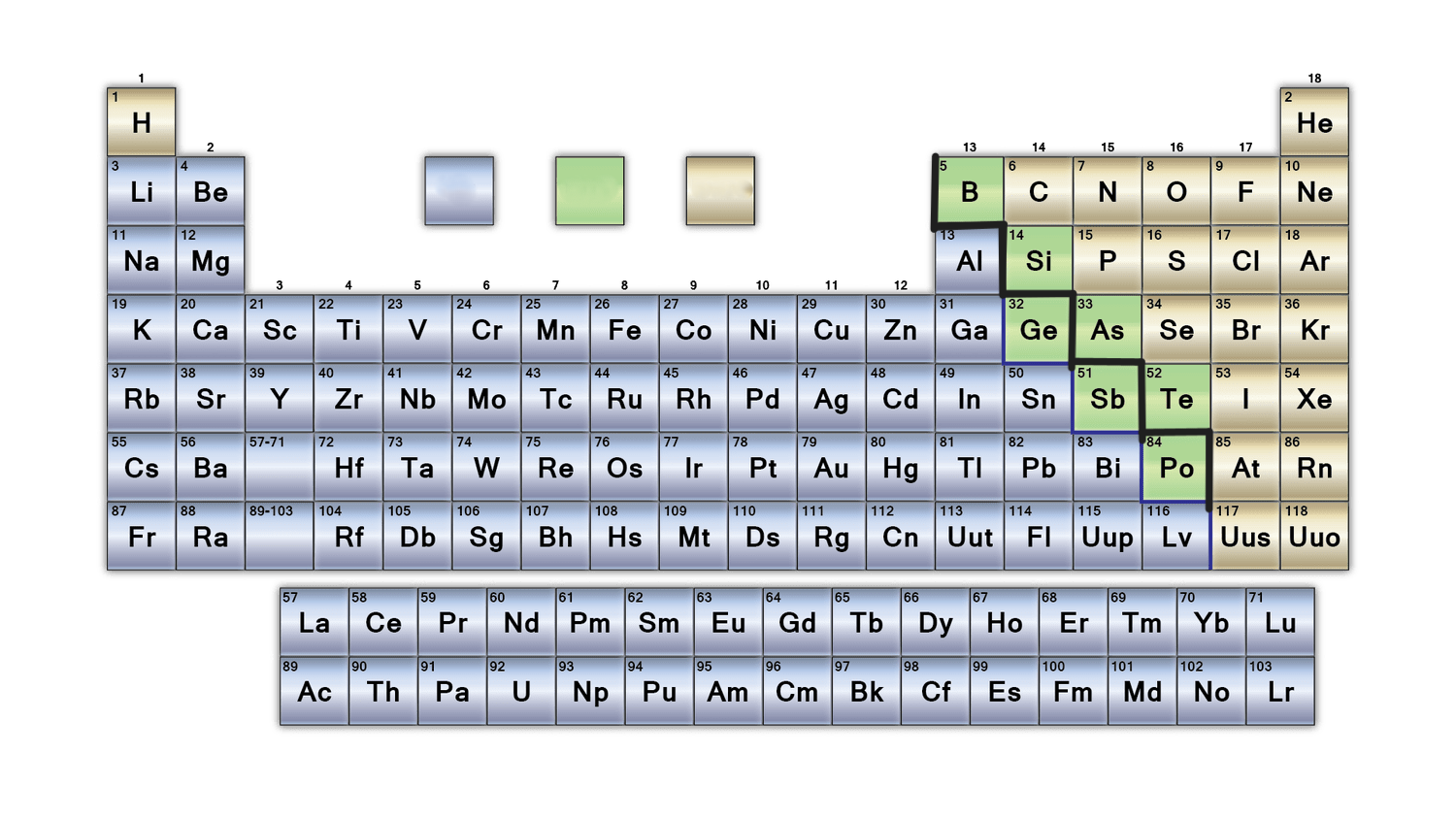
New cards
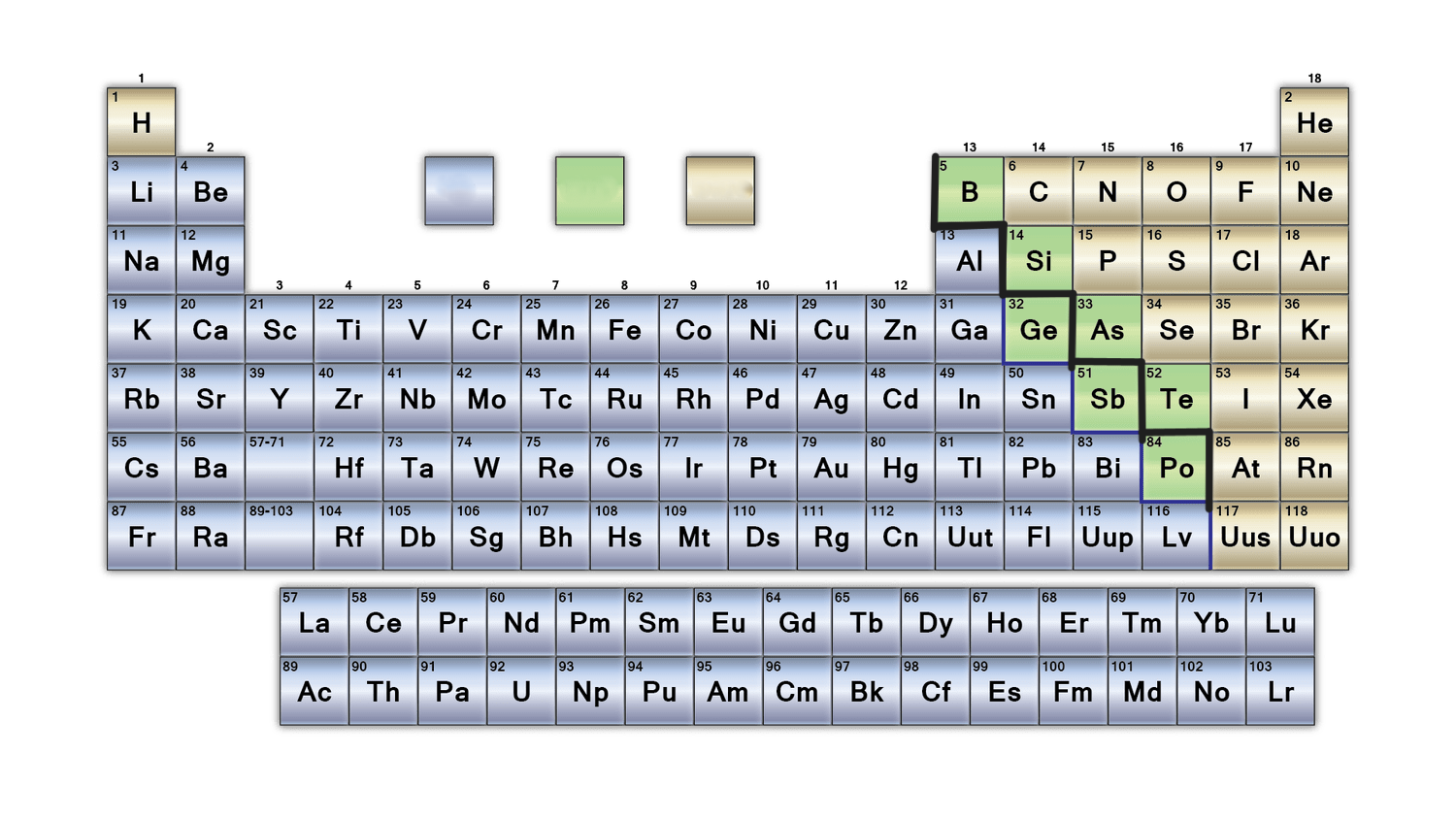
What is the purpose of the bold black line on the periodic table?
separates metals from nonmetals. Metalloids border this line
30
New cards
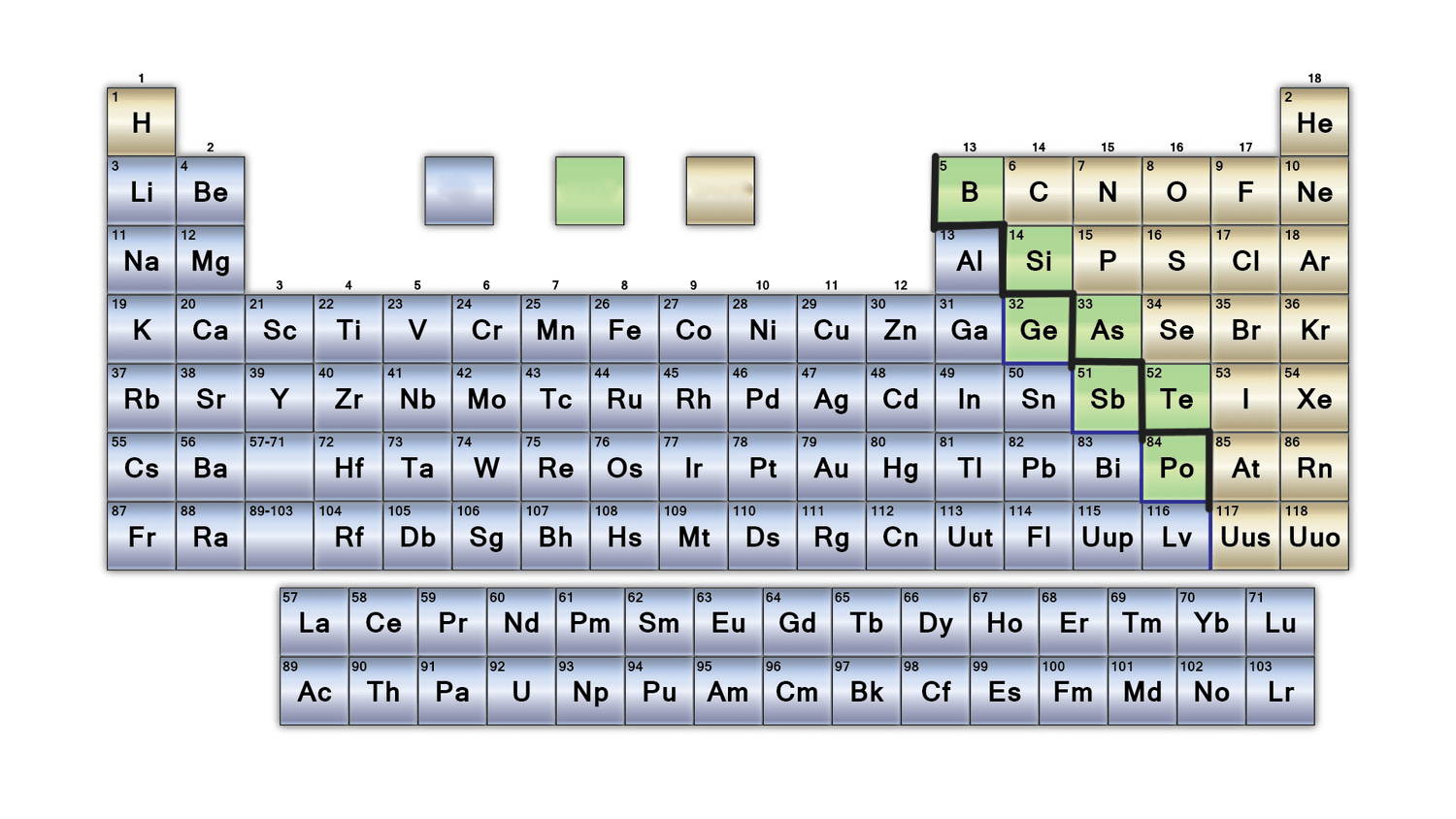
What is the zig zag line on a periodic table called?
Amphoteric line
31
New cards
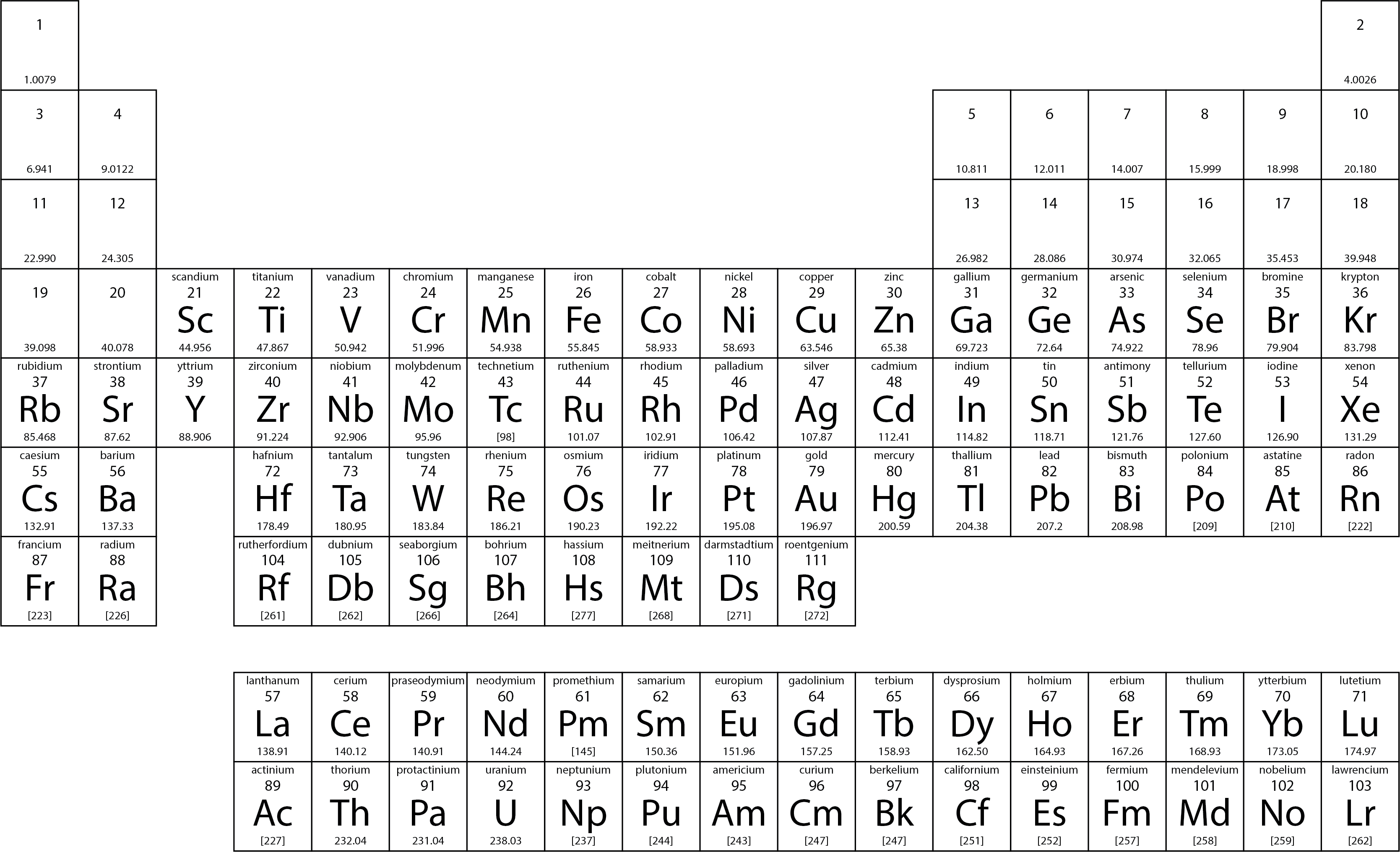
name the first 20 elements of the periodic table
hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, neon, sodium, magnesium, aluminium, silicon, phosphorus, sulphur, chlorine, argon, potassium, calcium
32
New cards
What are rows in the periodic table called?
Periods
33
New cards
How are periods arranged?
represent electron shells. Each period has a specific number of shells, from 1 to 7. Elements in a period have the same number of shells, but valence electrons increase from left to right.
34
New cards
What are columns in the periodic table called?
Groups
35
New cards
How are groups arranged?
Members of the same group have the same number of electrons in the outermost shell
36
New cards
define metal
any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light.
37
New cards
define non metal
a chemical element that lacks the characteristics of a metal
38
New cards
define metalloid
a chemical element that forms a simple substance having properties intermediate between those of a typical metal and a typical nonmetal.
39
New cards
how many metalloids are there?
6
40
New cards
list the metalloids
boron, silicon, germanium, arsenic, antimony and tellurium
41
New cards
how are metals and non metal elements different?
* Metals are solids at room temperature (except for mercury)
* metals are very hard (except for sodium) - non metals are soft (except for diamond)
* Metals are malleable and ductile - Non metals are brittle and can break down into pieces
* metals are shiny - non metals are non lustrous (except iodine)
* Metals are electropositive in nature - Non metals are electronegative in nature
* metals have high densities - non metals have low densities
* metals are very hard (except for sodium) - non metals are soft (except for diamond)
* Metals are malleable and ductile - Non metals are brittle and can break down into pieces
* metals are shiny - non metals are non lustrous (except iodine)
* Metals are electropositive in nature - Non metals are electronegative in nature
* metals have high densities - non metals have low densities
42
New cards
elements exist as or
atoms, molecules
43
New cards
what is the symbol for Hydrogen?
H
44
New cards
what is the symbol for Helium?
He
45
New cards
what is the symbol for Lithium?
Li
46
New cards
what is the symbol for Beryllium?
Be
47
New cards
what is the symbol for Boron
B
48
New cards
what is the symbol for Carbon?
C
49
New cards
what is the symbol for Nitrogen?
N
50
New cards
what is the symbol for Oxygen?
O
51
New cards
what is the symbol for Fluorine?
F
52
New cards
what is the symbol for Neon?
Ne
53
New cards
what is the symbol for Sodium?
Na
54
New cards
what is the symbol for Magnesium?
Mg
55
New cards
what is the symbol for Aluminium?
Al
56
New cards
what is the symbol for Silicon?
Si
57
New cards
what is the symbol for Sulphur?
S
58
New cards
what is the symbol for Chlorine?
Cl
59
New cards
what is the symbol for Argon?
Ar
60
New cards
what is the symbol for Potassium?
K
61
New cards
what is the symbol for Calcium?
Ca
62
New cards
what is the symbol for iron?
Fe
63
New cards
what is the symbol for mercury?
Hg
64
New cards
what is the symbol for silver?
Ag
65
New cards
what is the symbol for gold?
Au
66
New cards
what is the symbol for copper?
Cu
67
New cards
what is the symbol for lead?
Pb
68
New cards
what is the symbol for nickel?
Ni
69
New cards
what is the symbol for tin?
Sn
70
New cards
what is the symbol for platinum?
Pt
71
New cards
What is Sn the chemical symbol of?
Tin
72
New cards
What is Pb the chemical symbol of?
Lead
73
New cards
What is Hg the chemical symbol of?
Mercury
74
New cards
What is Ag the chemical symbol of?
Silver
75
New cards
What is K the chemical symbol of?
Potassium
76
New cards
How do elements get their symbols?
It often derived from the scientific name, but can also be from the Latin name
77
New cards
What is the formula for water?
H₂O
78
New cards
What is the formula for carbon dioxide?
CO₂
79
New cards
What is the formula for table salt?
NaCl
80
New cards
Using the following formula how many atoms are there on each side?
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
36
81
New cards
Using the following formula how many atoms of carbon are there on each side?
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
6
82
New cards
Using the following formula how many atoms of hydrogen are there on each side?
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
12
83
New cards
Using the following formula how many atoms of oxygen are there on each side?
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
18
84
New cards
Is this formula balanced?
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
Yes
85
New cards
is this formula balanced?
HCl + CaCO₃ → CaCl₂ + H₂O + CO₂
If not, balance the equation
HCl + CaCO₃ → CaCl₂ + H₂O + CO₂
If not, balance the equation
No
2HCl + CaCO₃ → CaCl₂ + H₂O + CO₂
2HCl + CaCO₃ → CaCl₂ + H₂O + CO₂
86
New cards
Why is it important to balance equations
Balanced equations follow the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction, only rearranged. Balancing equations ensures that the number of atoms of each element remains the same on both sides, indicating a balanced reaction. This helps scientists determine the quantity of reactants and products in a chemical reaction.
87
New cards
What do compounds exist as?
Molecules
88
New cards
what is the difference between physical and chemical changes
Physical changes involve altering the state or appearance of a substance without changing its chemical composition. Chemical changes result in the formation of new substances with different chemical properties.
89
New cards
Give an example of a chemical change
An example of a chemical change is the rusting of iron, where iron reacts with oxygen in the presence of moisture to form iron oxide.
90
New cards
Give an example of a physical change
An example of a physical change is the melting of ice into water.
91
New cards
What are the indicators of a chemical change?
* Colour Change.
* Production of an odour.
* Change of Temperature.
* Evolution of a gas
* Precipitate (formation of a solid)
* Production of an odour.
* Change of Temperature.
* Evolution of a gas
* Precipitate (formation of a solid)
92
New cards
The formula for photosynthesis is:
6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂
What are the reactants in this formula?
6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂
What are the reactants in this formula?
Carbon dioxide, water and energy
93
New cards
The formula for photosynthesis is:
6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂
What are the products in this formula?
6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂
What are the products in this formula?
glucose and oxygen
94
New cards
An example of a chemical change is the rusting of iron, where iron reacts with oxygen in the presence of moisture to form iron oxide.
Write a word formula for this equation
Write a word formula for this equation
iron + oxygen + water → iron oxide