KINETIC MOLECULAR THEORY
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
gases consist of tiny particles. the distance is larger than the size of particles themselves.
no attractive force exists, particles occupy all available space
moves in a linear motion but change direction upon collision. they collide frequently and are perfectly elastic
average kinetic energy is the same for all gases at a given temp, and its value is directly proportional to the kelvin temperature
kinetic molecular theory
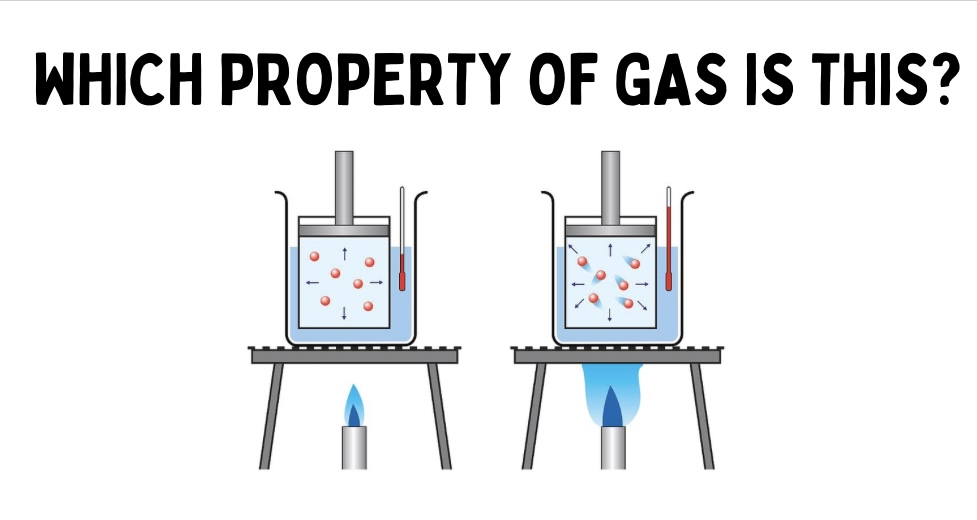
temperature
measure of kinetic energy of gas particles
volume
amount of space occupied by gas particles
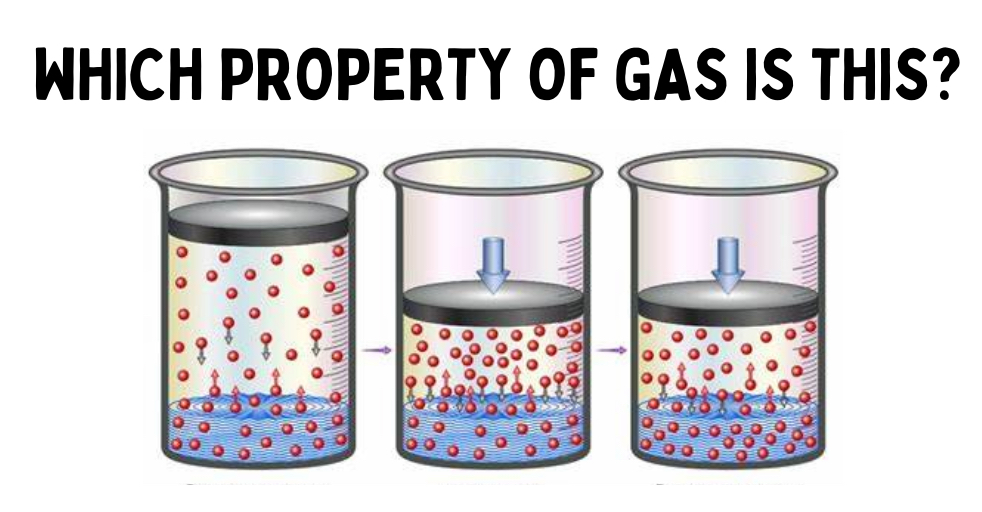
pressure
amount of force exerted per unit area. gases exert force to its container
barometer
instrument to measure atmospheric pressure
atmospheric pressure
composed of gases that exerts force. denser close to earth and less dense afar.
Pressure
Temperature
Volume
