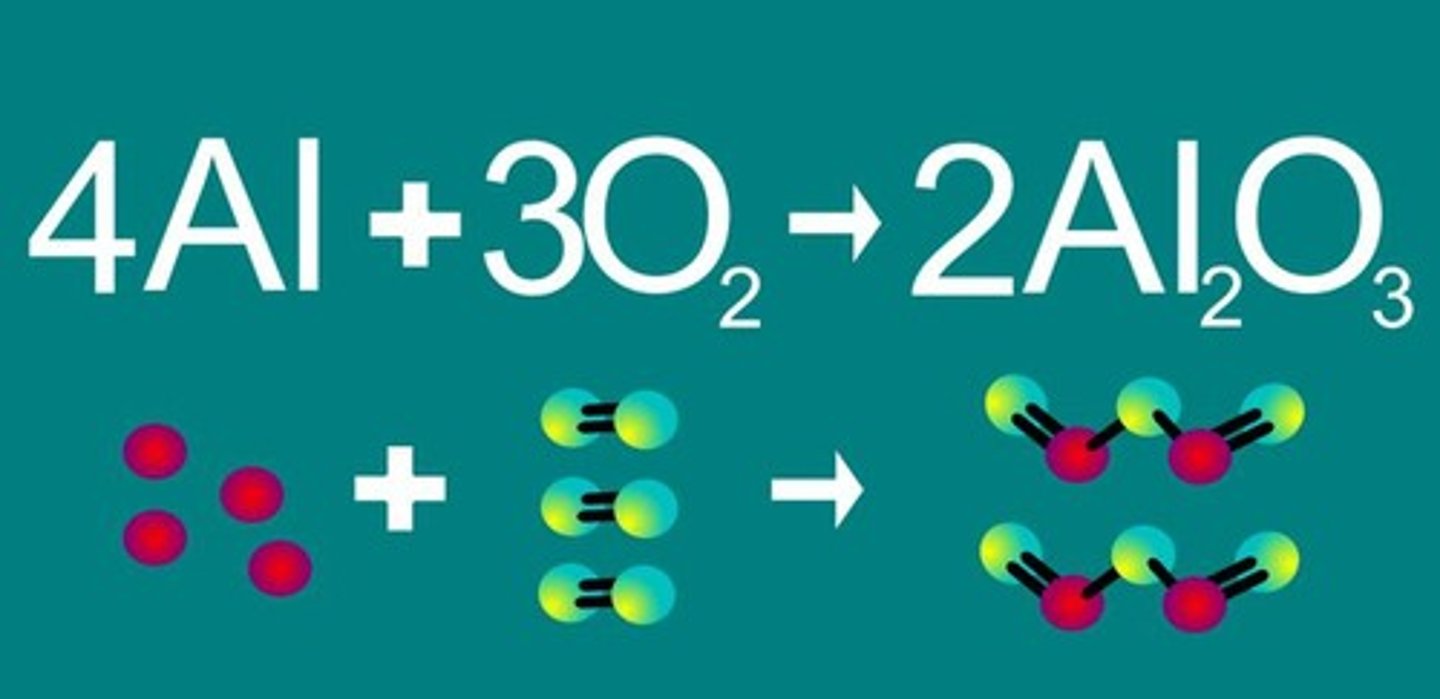Basic Chemistry terms
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Chemical Energy
________can hold matter together or break it apart.
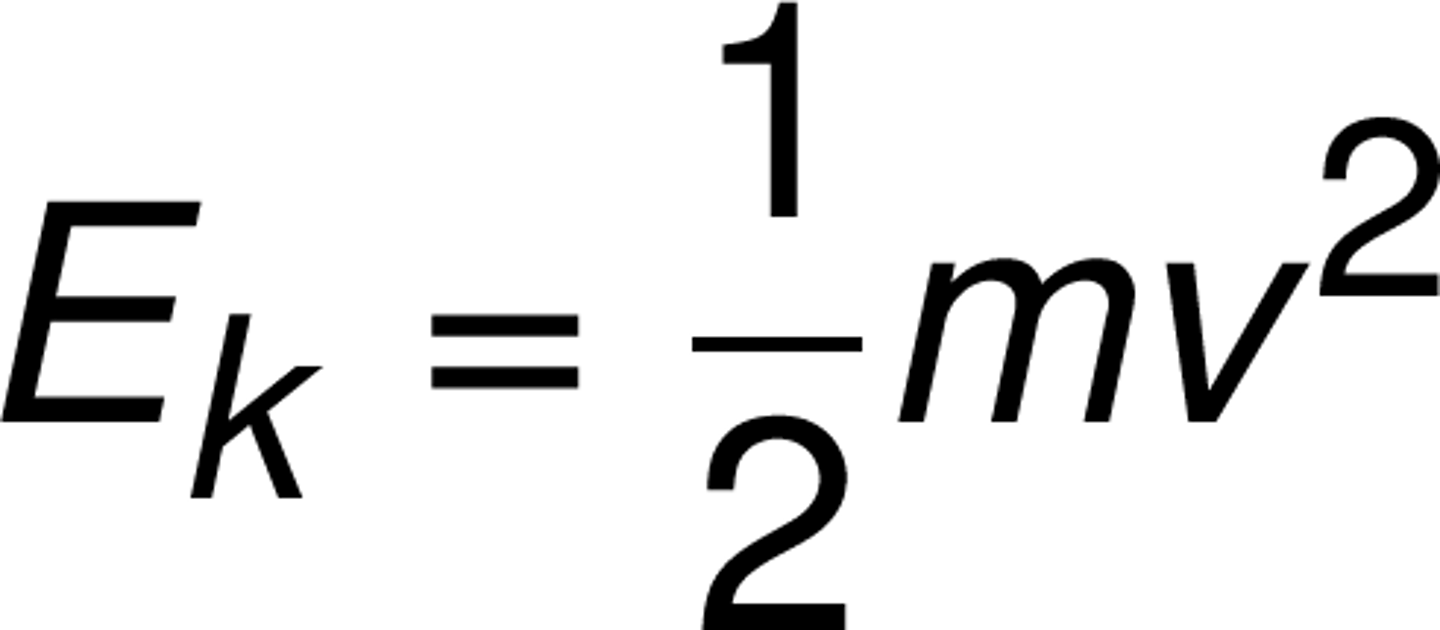
Matter
Anything that has mass and takes up space

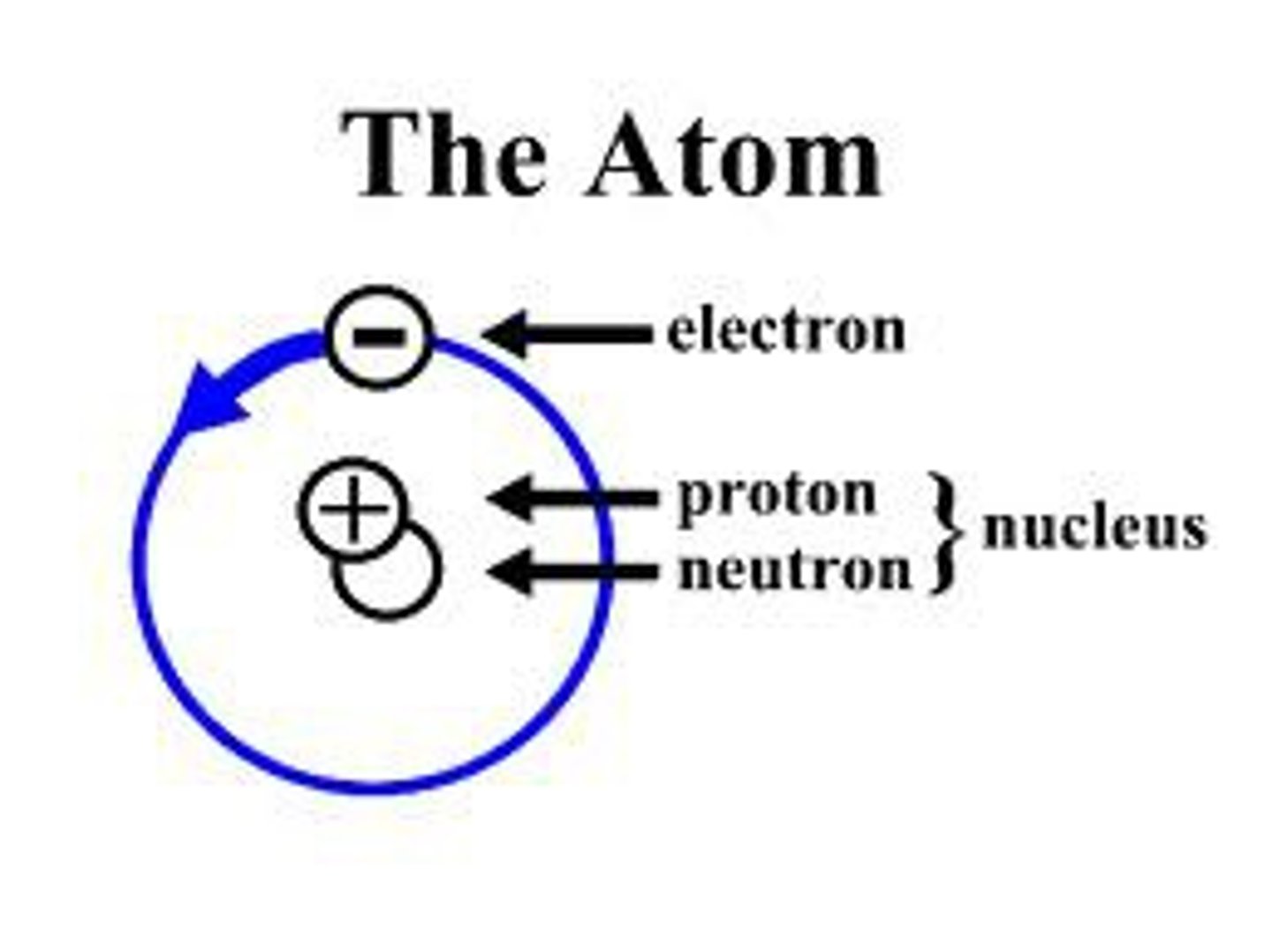
Atom
Basic unit of matter. The smallest part of an element.
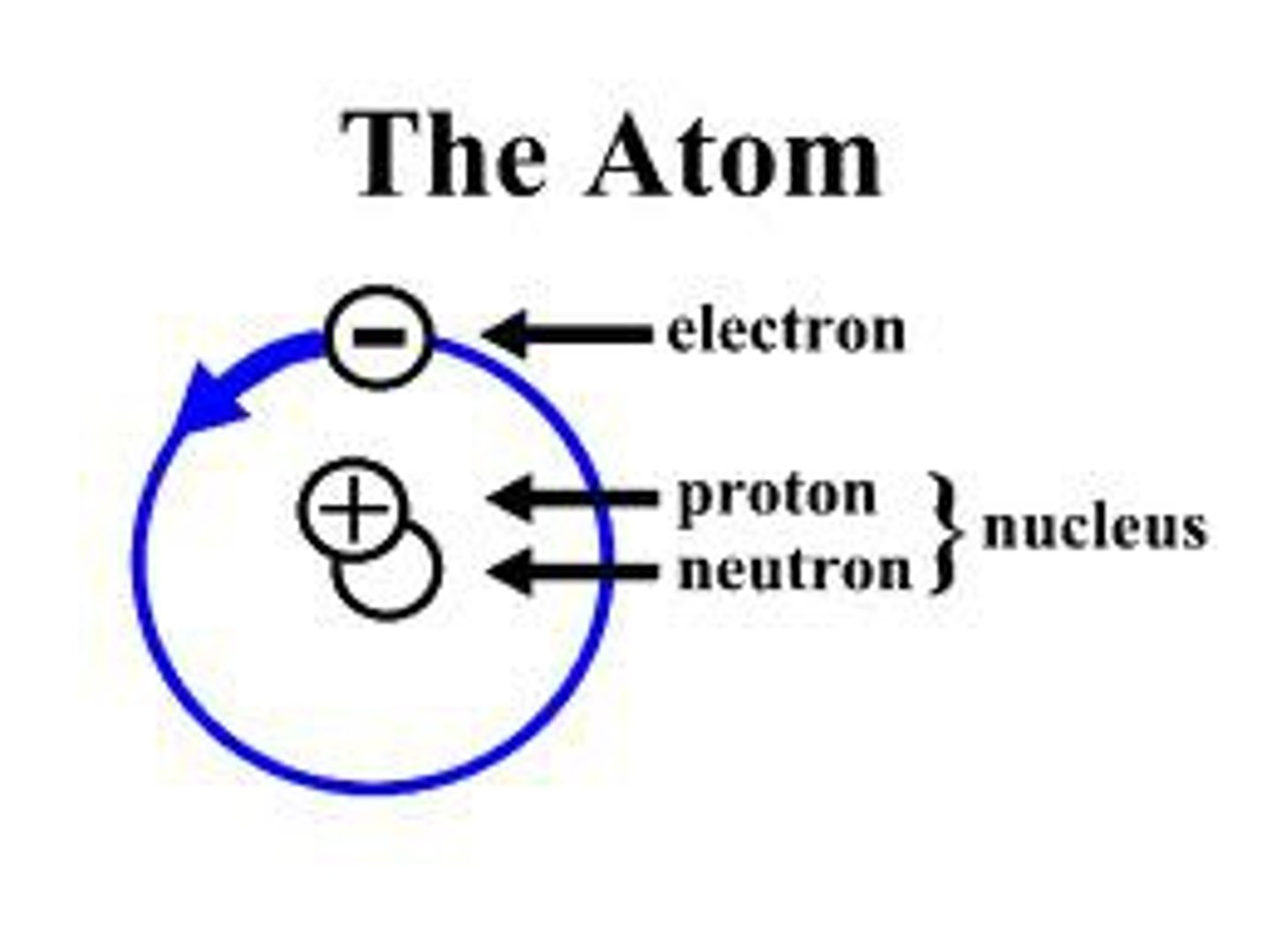
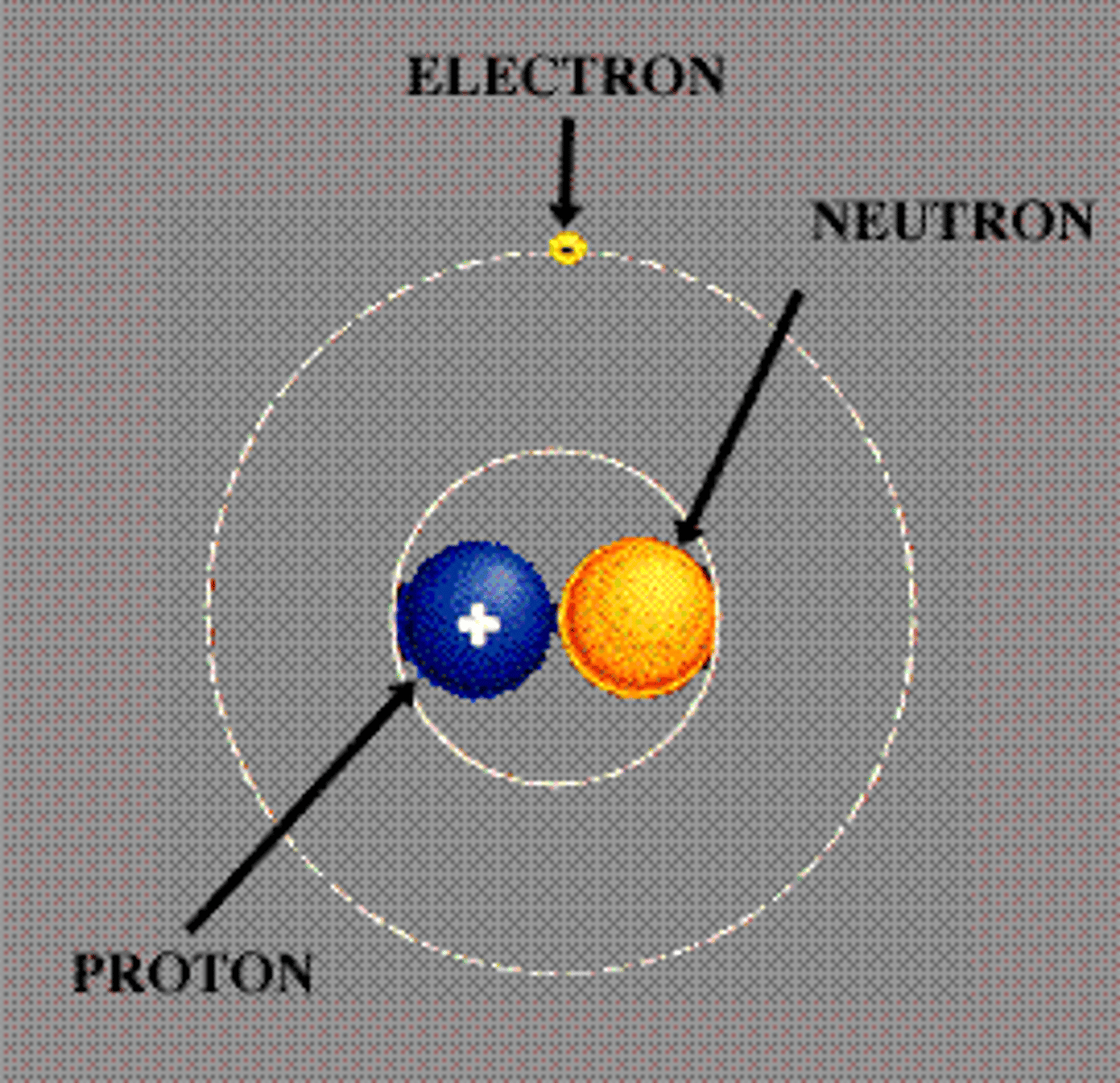
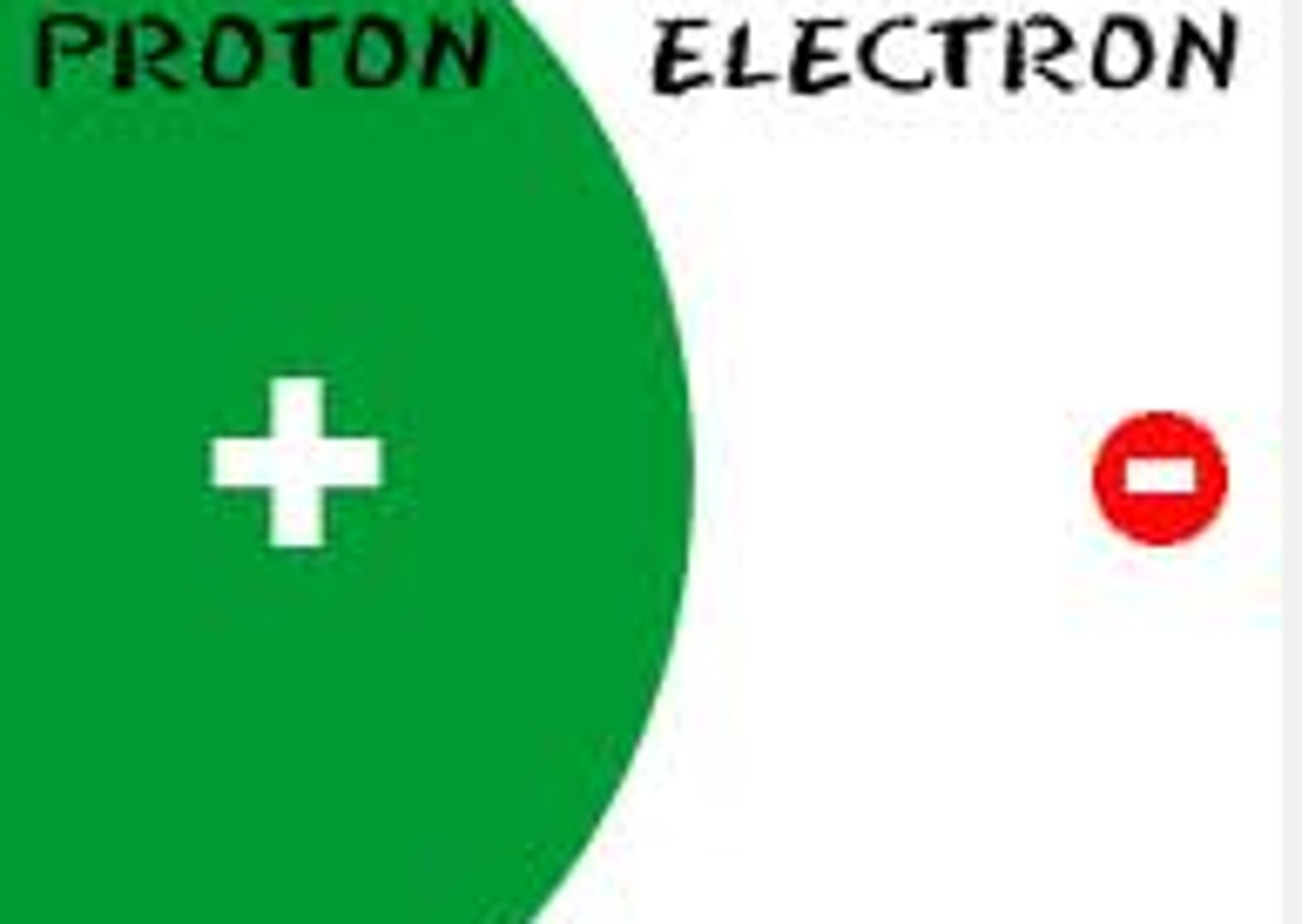
Proton
The positive part of the atom.
Electron
The negative part of the atom is
Neutron
The neutral part of the atom
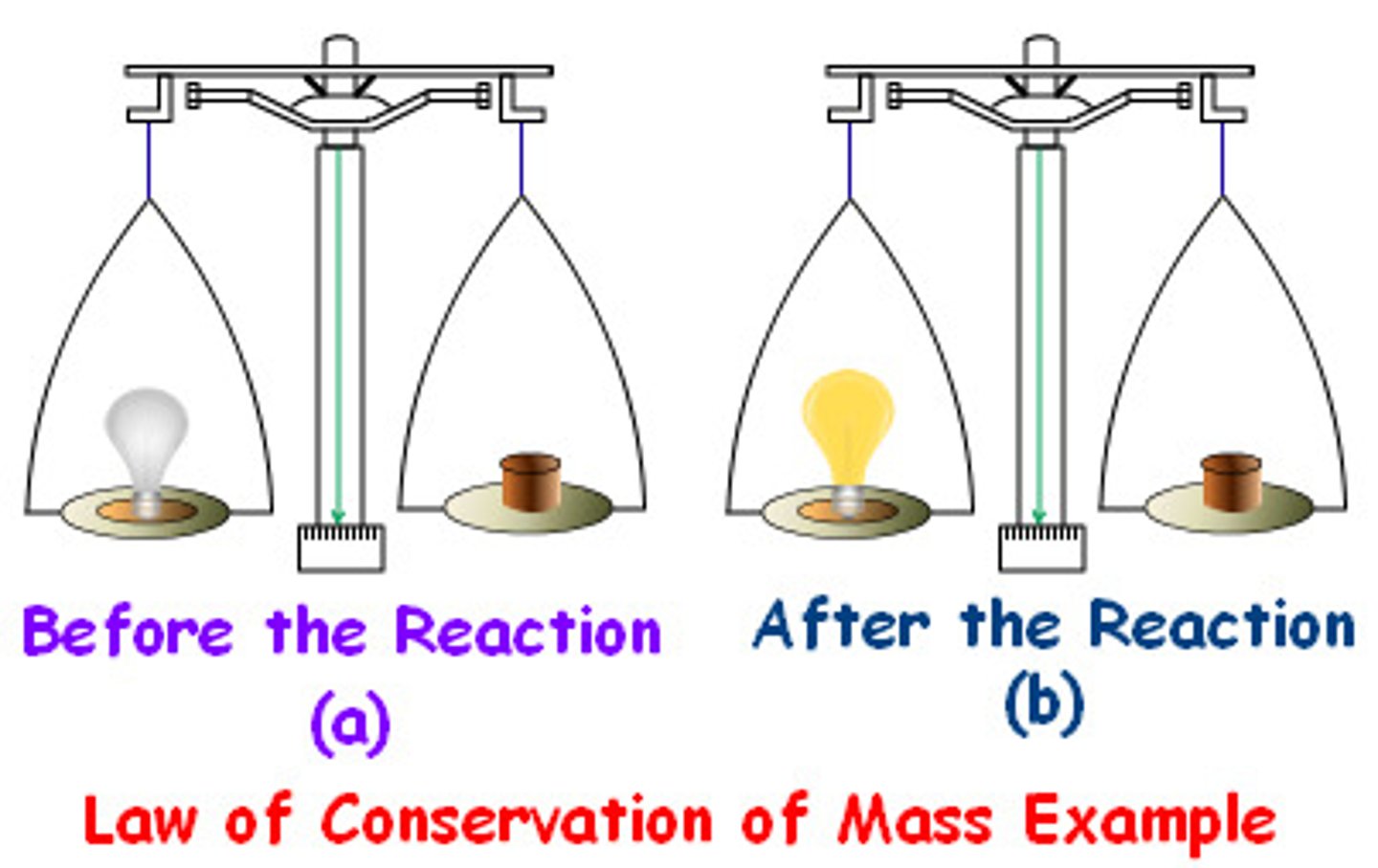
Law of conservation of matter states. . .
Matter cannot be created nor destroyed, but only changed in form.
Element
Substance that cannot be broken down into simpler substance (one kind of atom)
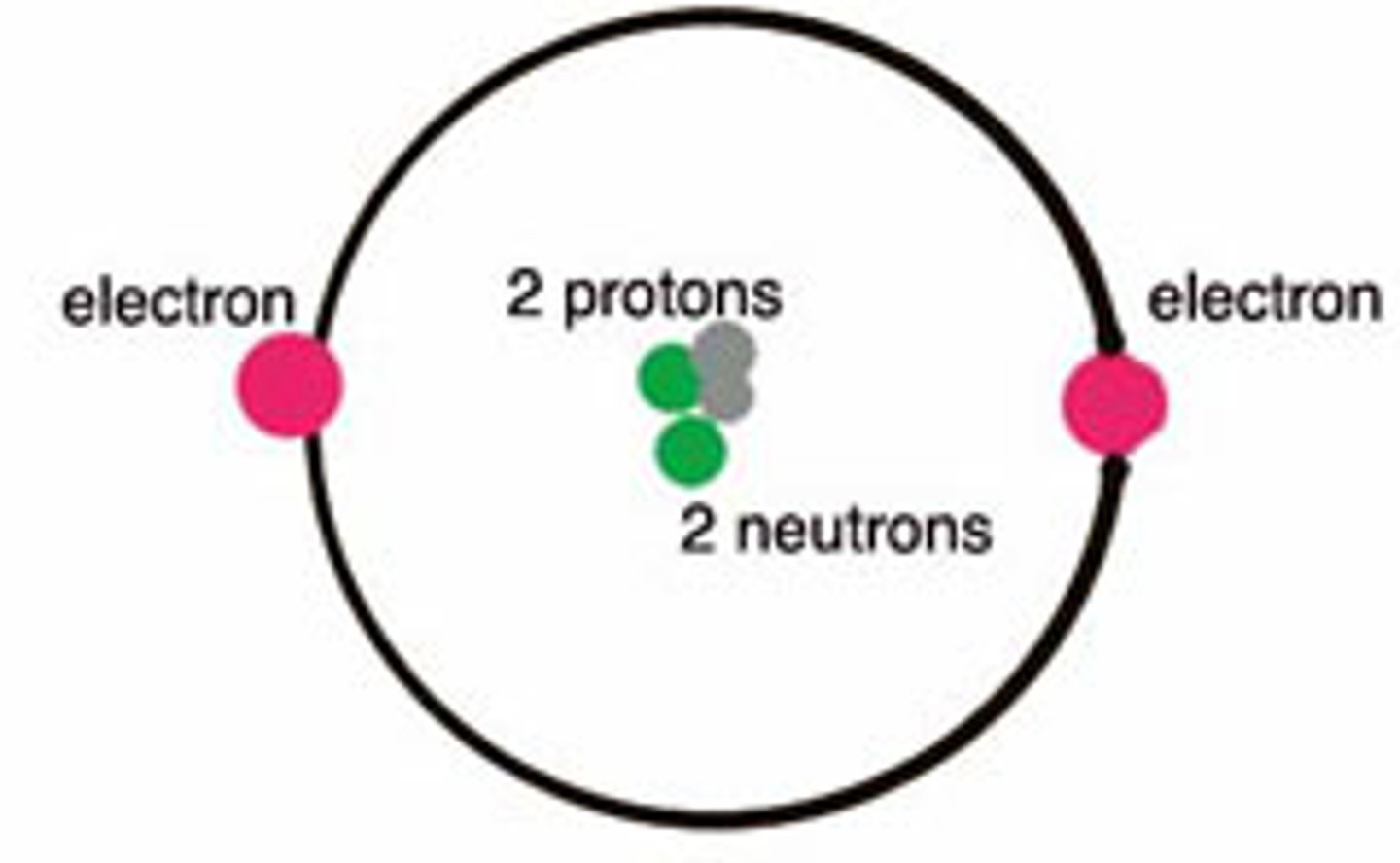
Protons and Neutrons
The nucleus of an atom contains the . . .
The valence electrons are involved with _____________
Chemical bonding
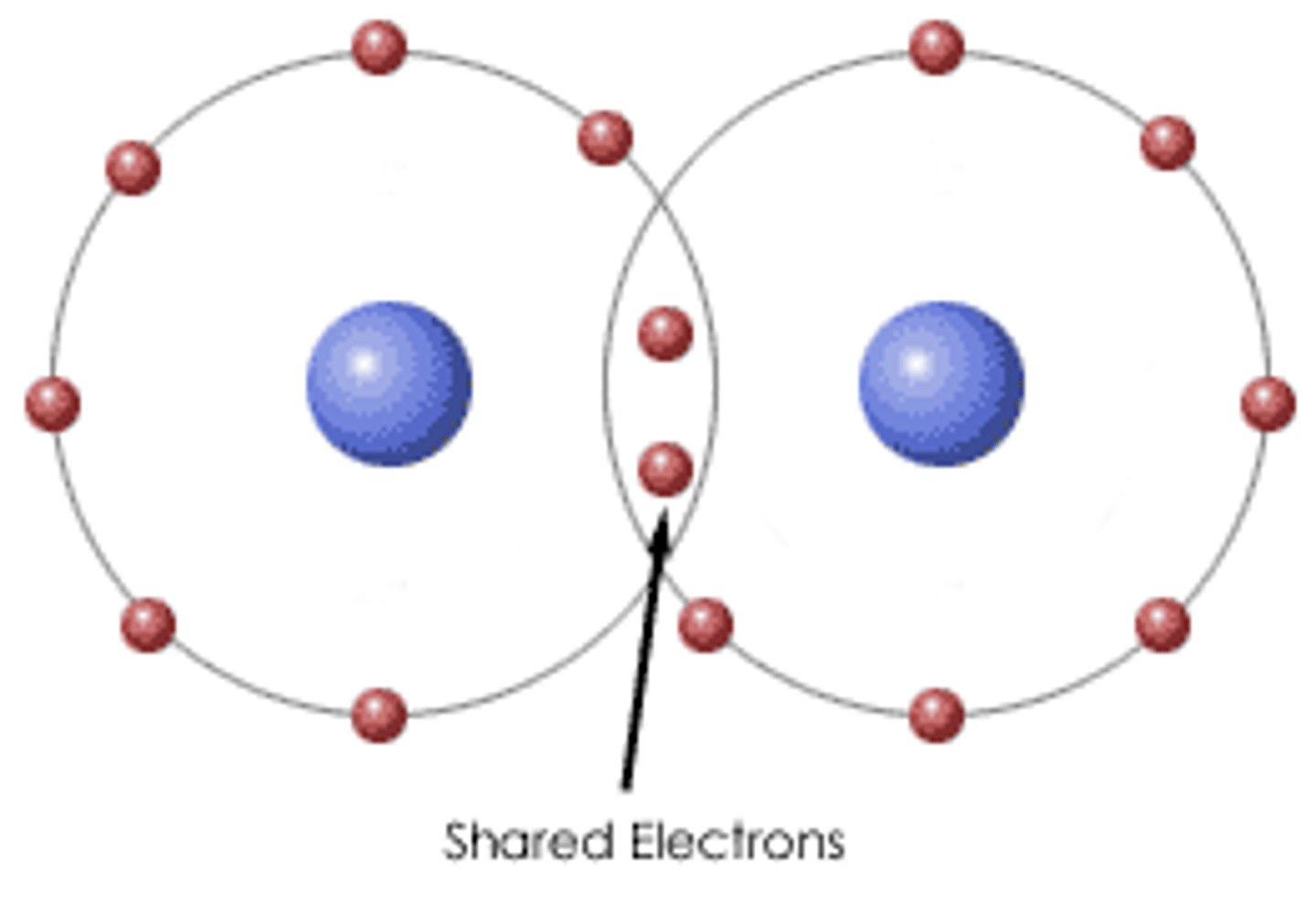
________ are made of two or more elements bonded in exact proportions
Molecular compounds
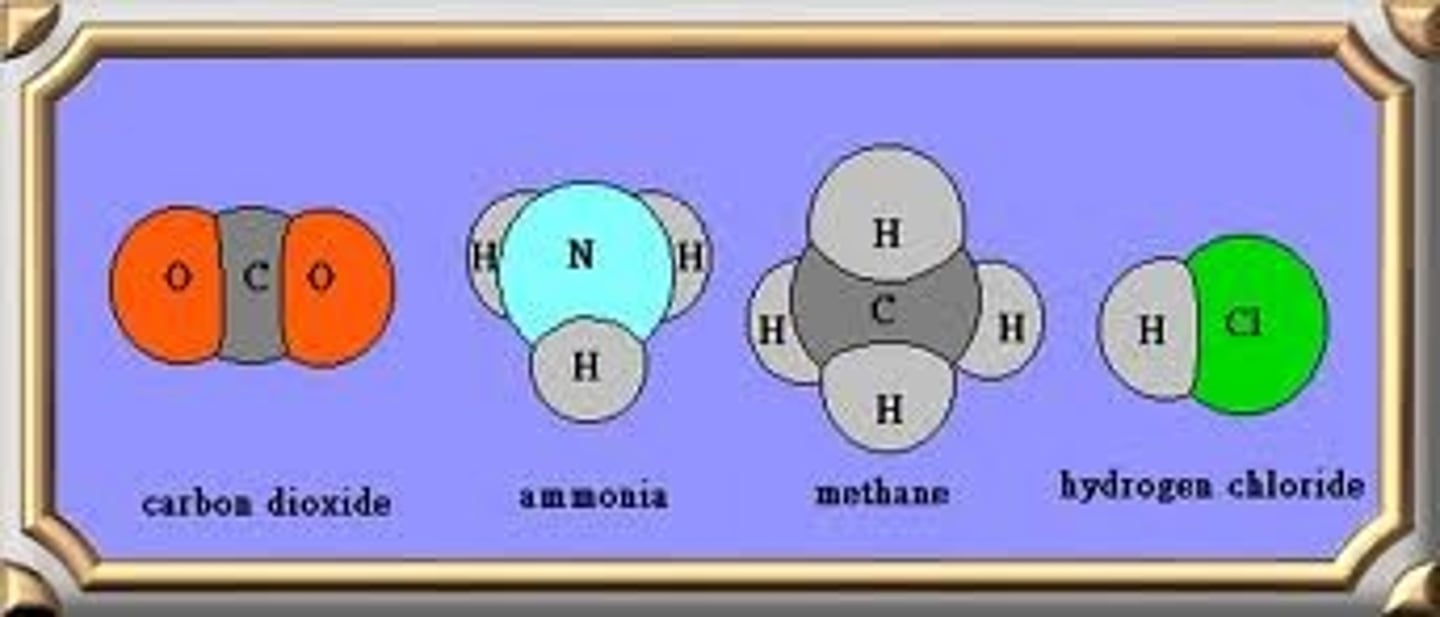
The smallest part of a substance with characteristics of that substance.
Molecule

Mixture
2 or more substances mixed but not chemically combined

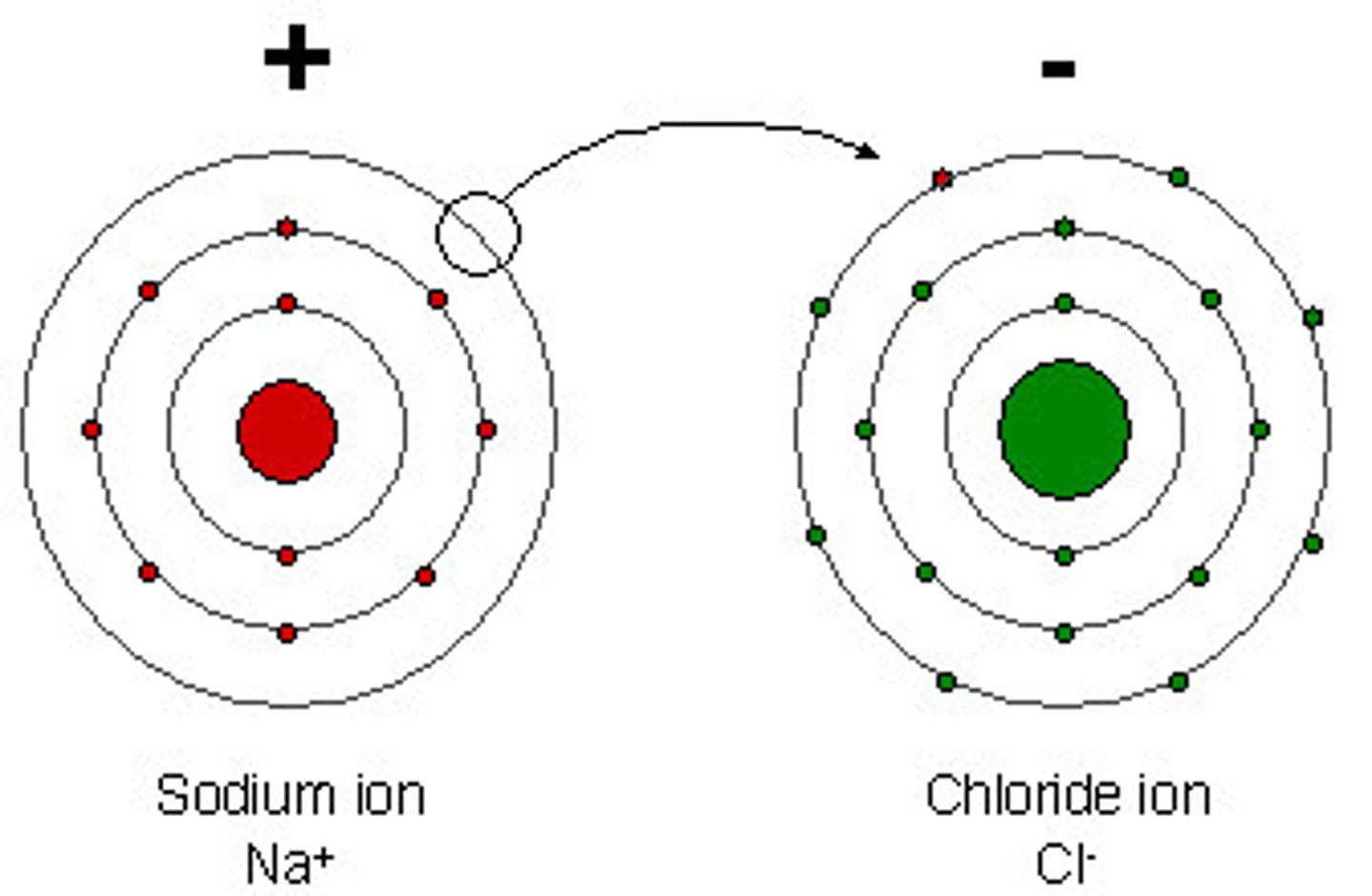
Ions
Atoms that have an electrical charge are ________
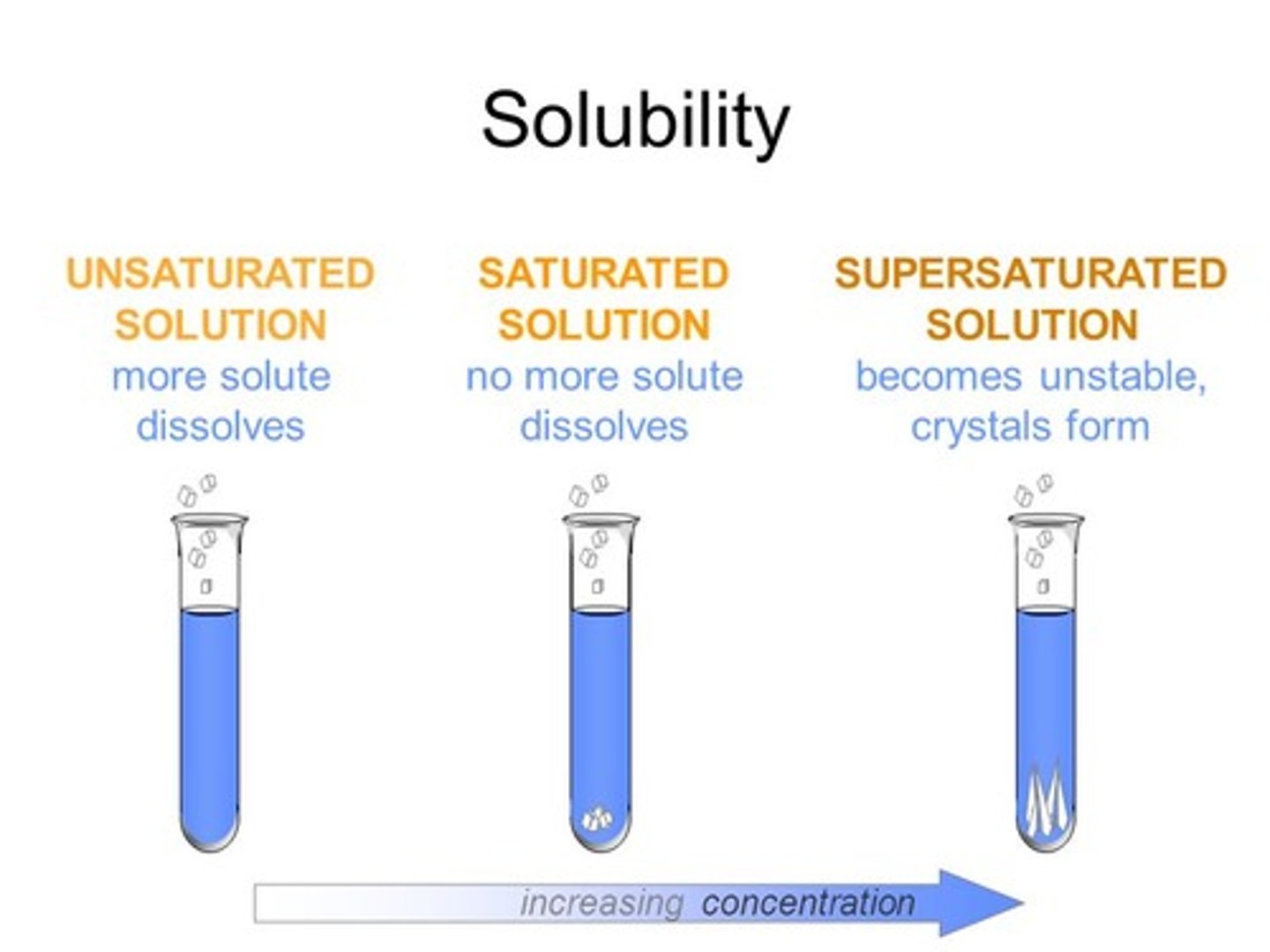
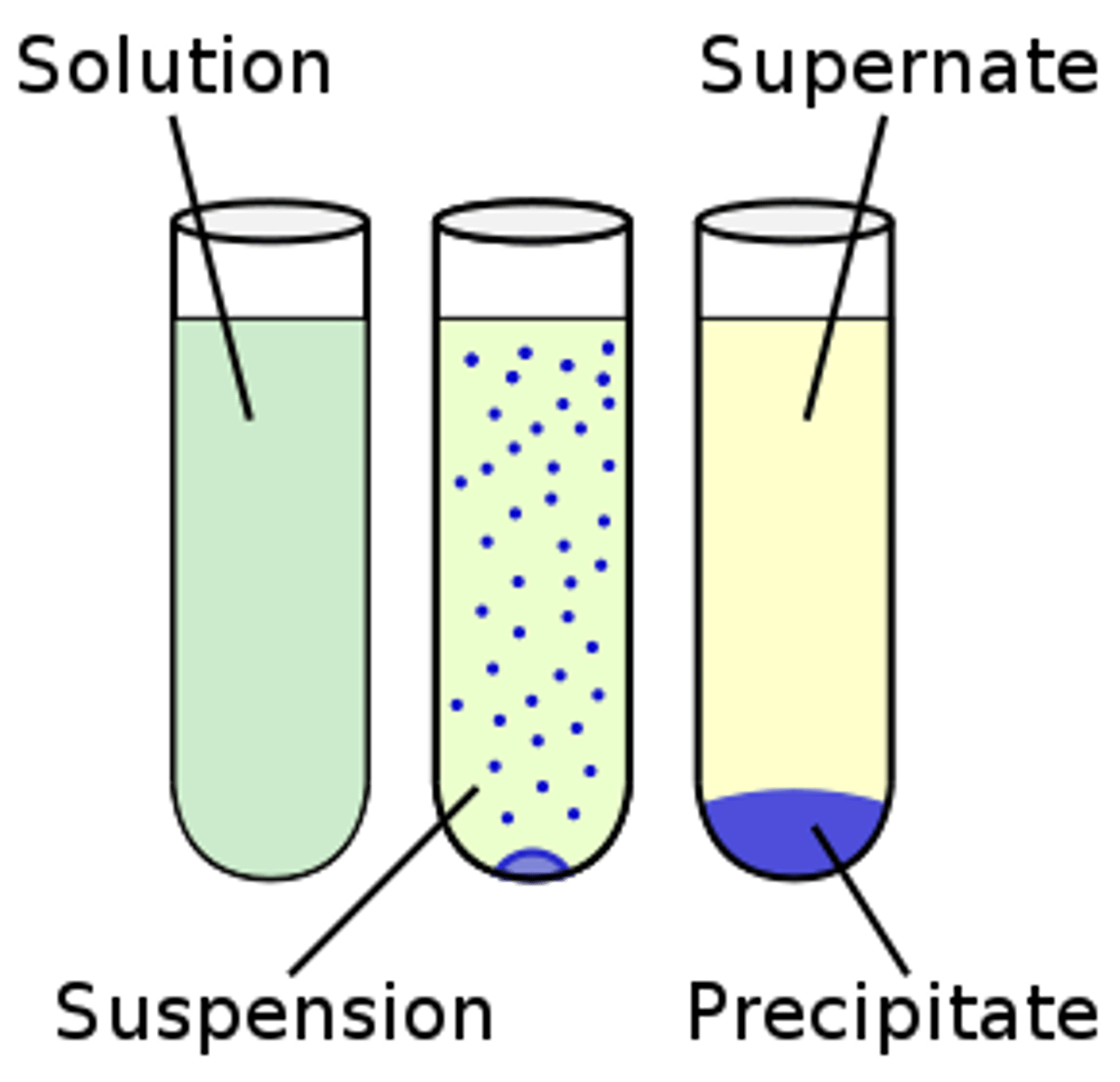
Solution
homogeneous molecular mixture containing two substances having the same properties throughout the mixture.
Suspension
Heterogenous mixture of particles that will eventually settle out.
Atomic mass
Sum of neutrons and protons in an atom (when rounded to nearest whole number)
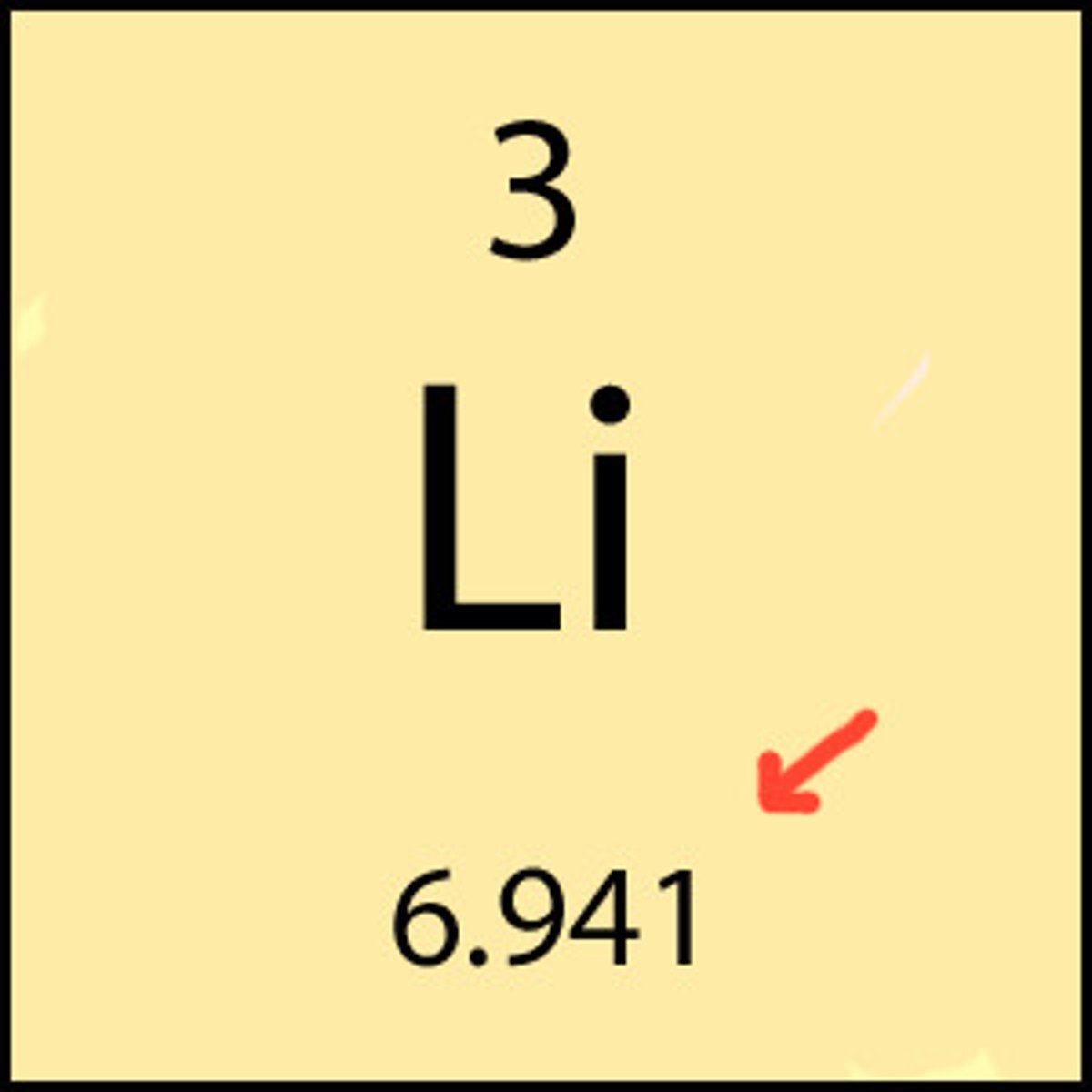
Atomic number
Number of protons in an atom.
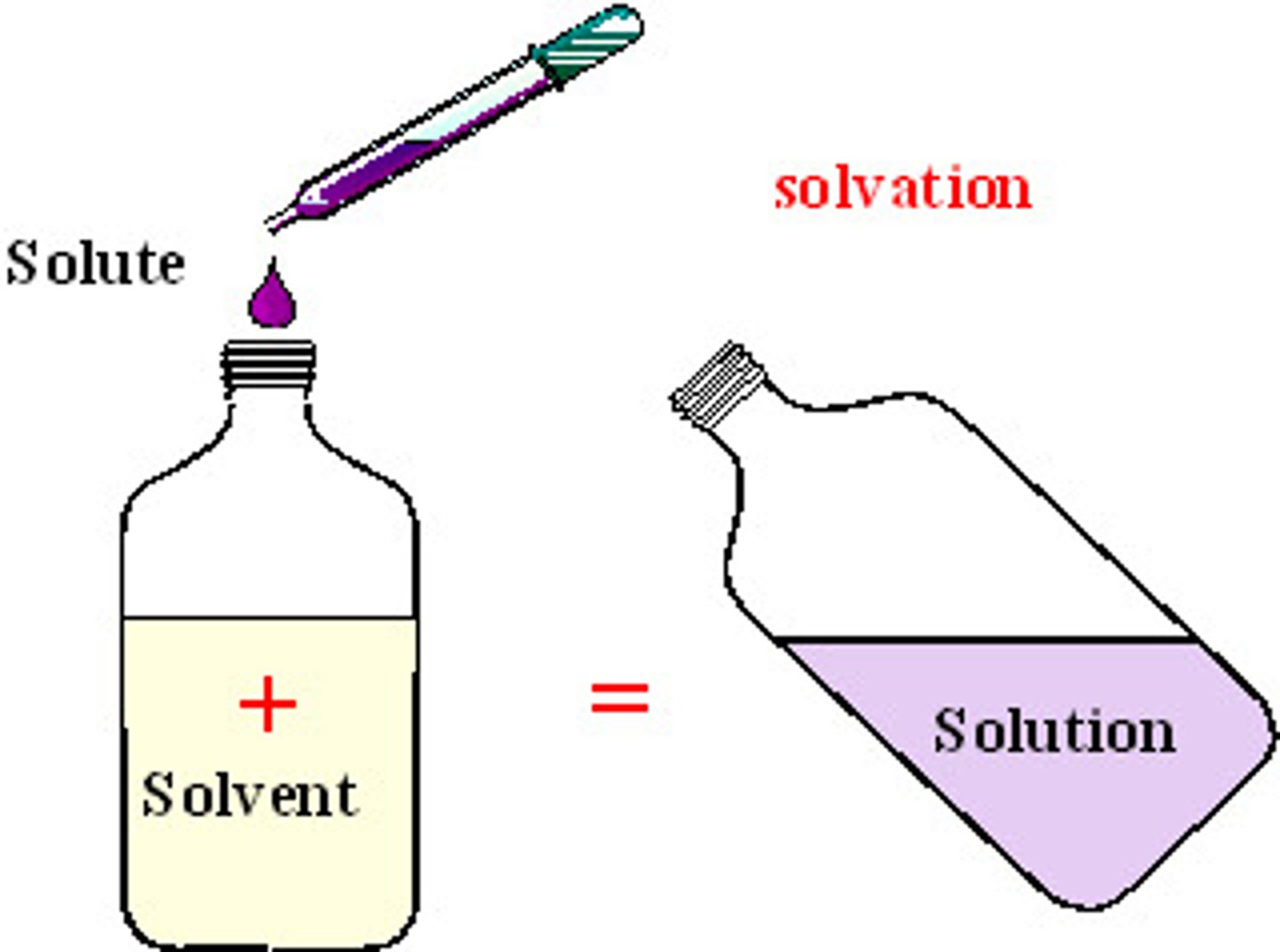
solute
substance being dissolved in solution
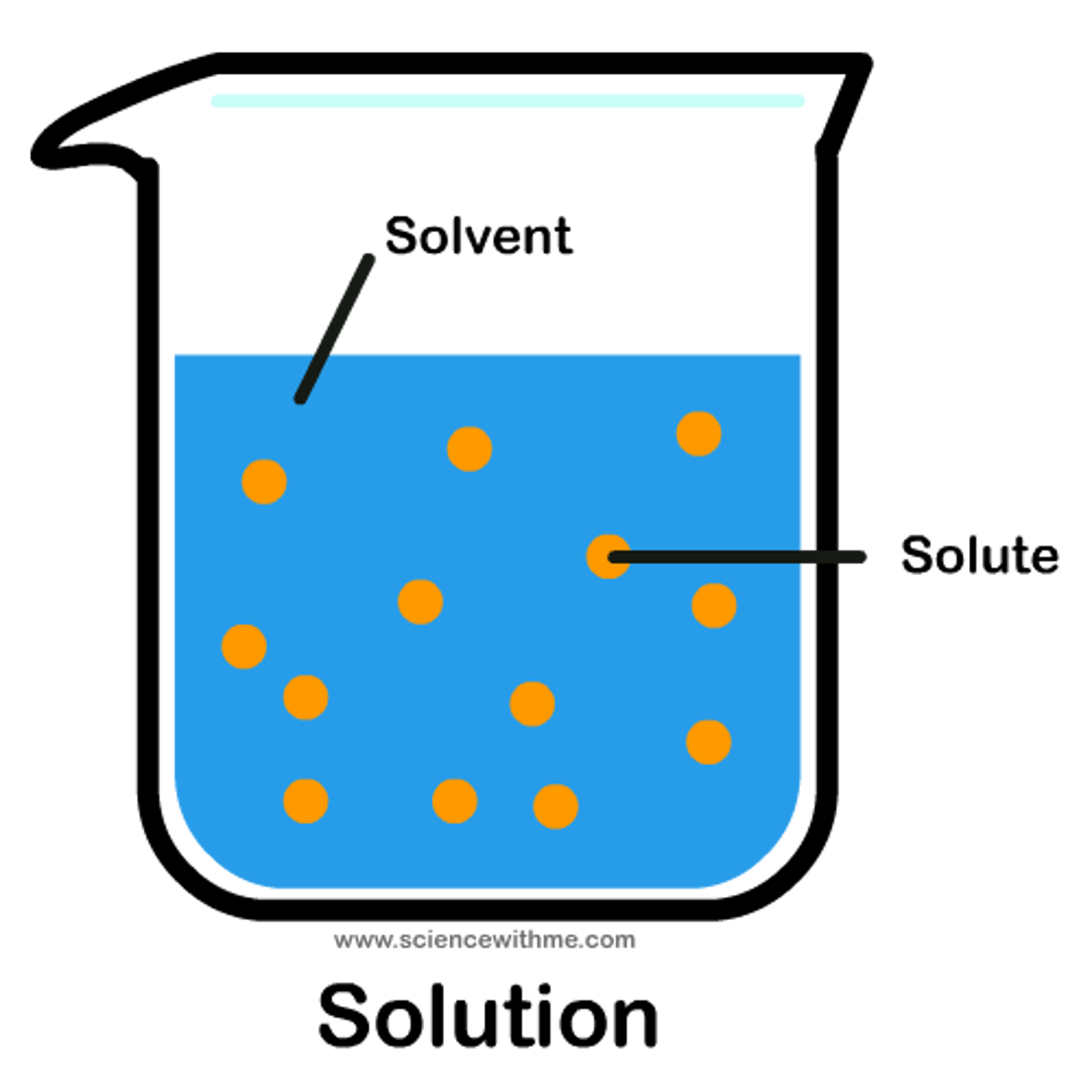
solvent
substance that does the dissolving
organic
substance that was living at one time
inorganic
something that was never alive

homogeneous
of the same kind; similar; uniform

heterogeneous
Composed of unlike parts, different, diverse

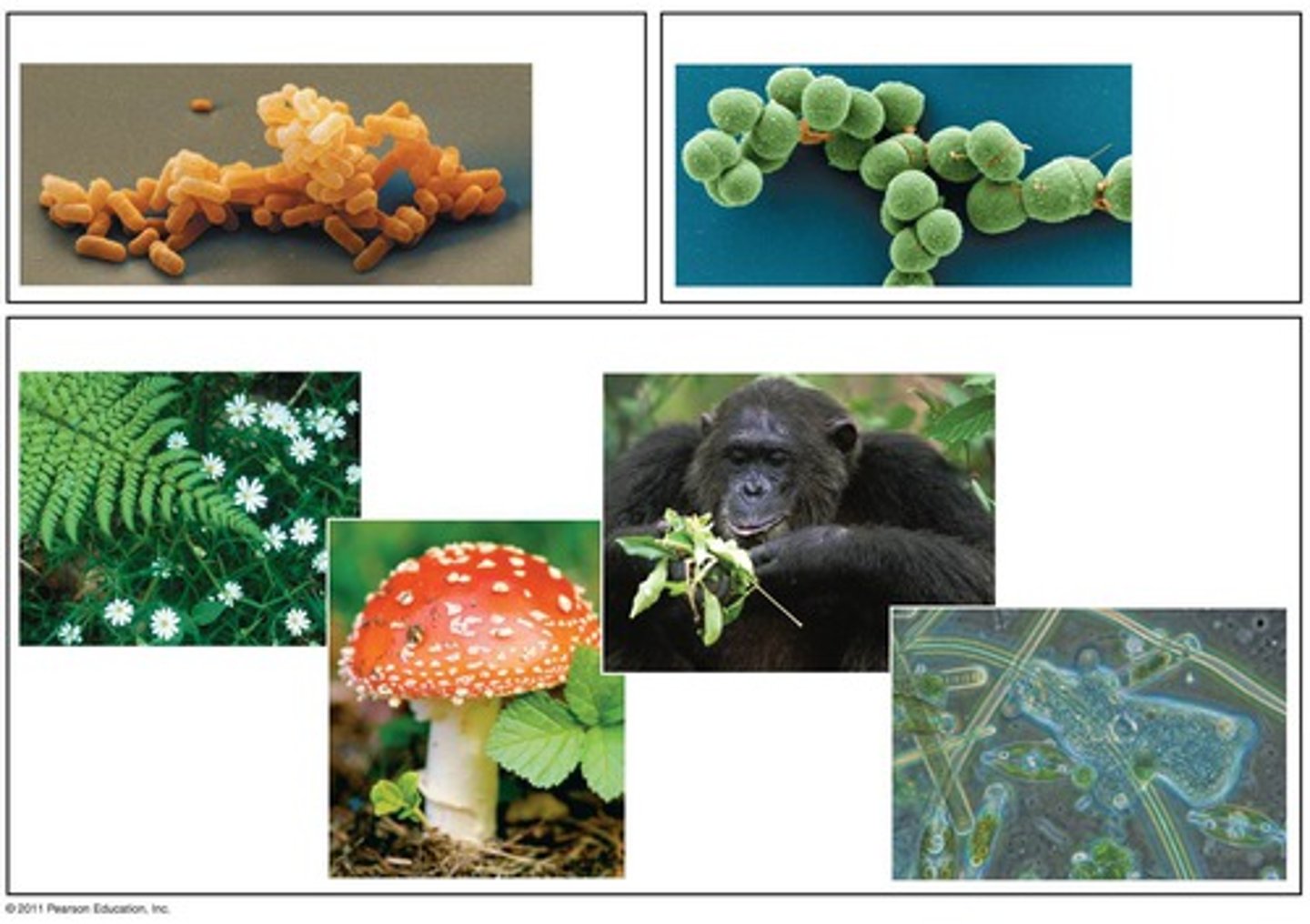
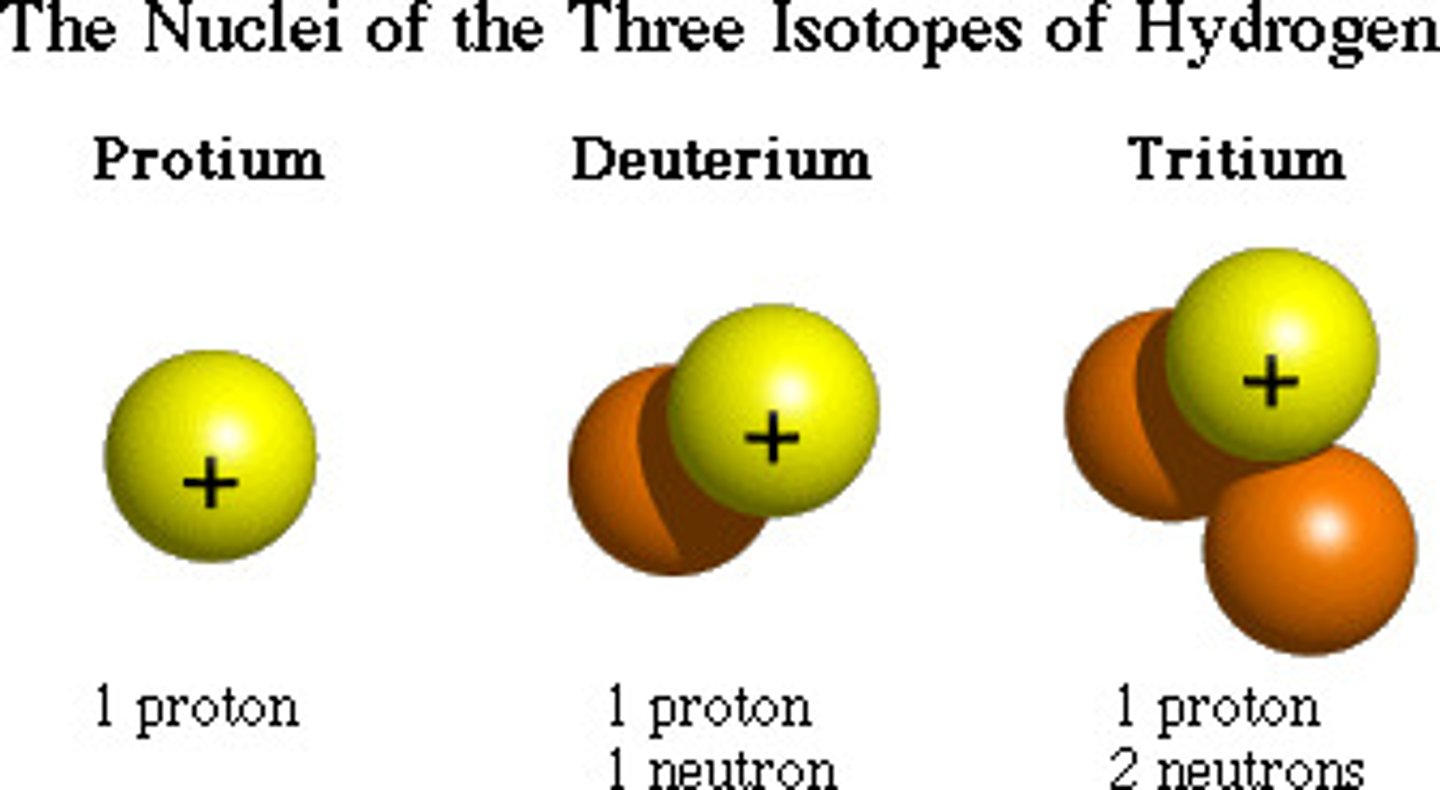
isotope
atoms of the same element (equal numbers of protons) but different numbers of neutrons (example Carbon 14 and Carbon 12)
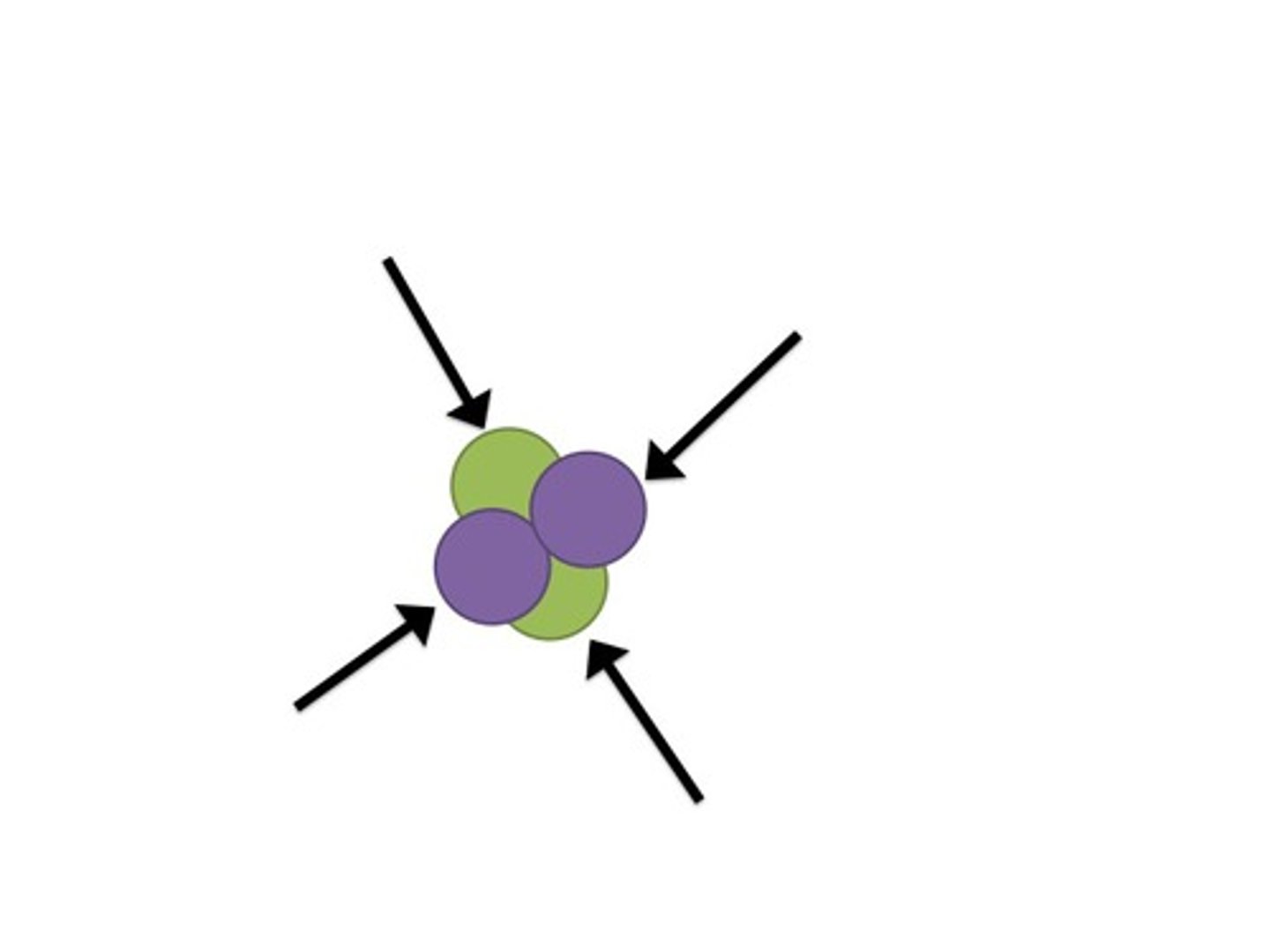
Strong Force
interactions within the nucleus of an atom that hold its nucleus together; strongest force known
physical change
A change in a substance that does not change its identity
energy
The ability to do work or cause change
nutrient
chemical substance that an organism needs to sustain life
chemical change
substance changes to new substance that involves changes in the arrangement of atoms, molecules or ions.