Chapter 13 - The Atomic Nucleus and Radioactivity
1/18
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Which of the following nuclear equations correctly describes emission?
none of the above
If a nucleus of 232/90 Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons), what nucleus results?
uranium-233
Which of the following statements regarding a nucleon is true?
all of the above; the mass of a nucleon depends on which nucleus it is in, some of the mass of a nucleon can be converted into energy by breaking certain nuclei, attraction between nucleons changes their mass, both first and third choices
Radioactivity is a tendency for an element or a material to ____ ___
emit radiation
If carbon-14 is a beta emitter, what is the likely product of radioactive decay?
nitrogen-14
People who work around radioactivity wear film badges to monitor the amount of radiation that reaches their bodies. These badges consist of small pieces of photographic film enclosed in a light-proof wrapper. What kind of radiation do these devices monitor?
gamma radiation
The isotope Cesium-137, which has a half-life of 30 years, is a product of nuclear power plants. How long will it take for this isotope to decay to about one-sixteenth its original amount?
120 years
If uranium were to split into three segments of equal size instead of two, would more energy or less energy be released?
More energy would be released because of less mass per nucleon.
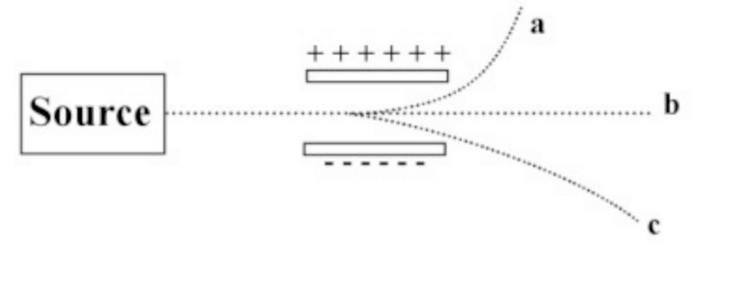
The image below shows a beam of radiation passing between two electrically charged plates.
Which of the beams is due to an energetic light wave?
b
Why would you expect alpha particles to be less able to penetrate materials than beta particles of the same kinetic energy?
two of the above answers are reasonable
Just after an alpha particle leaves the nucleus, would you expect it to speed up? Why?
Yes. Once the alpha particle leaves the nucleus it accelerates because of mutual electric repulsion with the nucleus from which it escaped.
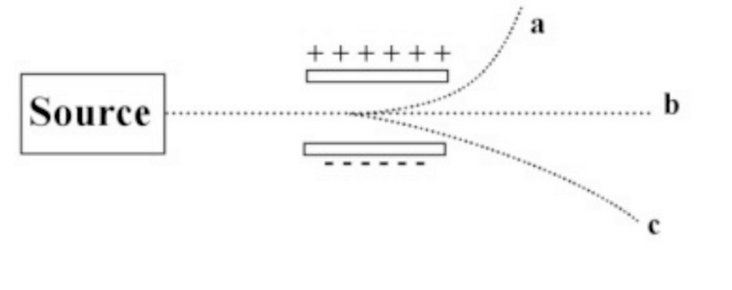
The image below shows a beam, of radiation passing between two electrically charged plates.
Which of the beams is due to a high energy electron?
A
Why does plutonium not occur in appreciable amounts in natural ore deposits?
Any plutonium initially in the earth's crust has long since decayed.
A neutron makes a better nuclear bullet than a proton or an electron because it
carries no electrical charge
which type of radiation results in the least change in atomic number?
gamma radiation
Why will nuclear fission probably not be used directly for powering automobiles?
all the above are true
Where does the earth recieve most of its energy?
external fusion
Which of the following statements regarding a nucleon is true in regards to nuclear reactions?
A nucleon inside a nucleus has a lower mass than a nucleon outside of the nucleus.
Which of the following is a major advantage of nuclear-fission-based power plants?
first and third choices; the amount of energy per kilogram of fuel is greater tan any other fuel source, the reactors can be used to produce their own fuel