Unit 1 Cell and Molecular Biology
1/134
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
135 Terms
What is biology the study of?
Living systems.
What are prokaryotes?
Bacteria and Archaea domains (they are extremophiles).
What are eukaryotes?
Plants, fungi, animals and protists.
Linnaean taxonomy
Carl Linnaeus created this, a systematic way to organize organisms into taxa. Organize plus categorize organisms in taxa based on morphological and structural features. Can be hard to classify when you can't see something.
What does “pro” mean in prokaryotes?
Before.
What does “eu” mean in eukaryotes?
True or good.
What does “karyon” mean in prokaryotes and eukaryotes?
Nut or kernel (nucleus).
What is basic research?
Uses some type of organism/system to learn biology.
What is translational research?
Finding questions/similarities translating to different organisms.
What is clinical research?
Treatment to increase lifespan/health span.
What do typical model organisms have?
Rapid Growth and development.
The ability to be easily manipulated (genetically etc).
Have a short lifespan.
Produce a large number of offspring.
Have a sequenced genome (this is relatively new).
Being well understood.
Prokaryotic workhouse
E.coli: DNA/RNA protein is conserved between humans and E.coli
Helps understand the genetic code (DNA).
Protein production and expression.
The first recombinant DNA technologies (taking bits and pieces of DNA and combining them).
Initial use for synthetic biology
Eukaryote system
Saccharomyces cerevisia (brewers yeast): Yeast bubbles and doubles in size, CO2 as a byproduct.
First eukaryote to be sequenced.
Around 23% homologous genes when compared to humans.
Applications include:
Aging
Gene Expression
Signal Transduction
Cell Cycle
Metabolism
Technology Development (DNA sequencing, ribosome profiling etc.)
Model Plant
Arabidopsis thaliana: Thale cress, a weed.
Genome maintenance and regulation.
Epigenetics.
miRNA and gene expression.
First plant genetically sequenced.
All things plant.
Drosophila melanogaster
A fruit fly:
Around 75% of the genes that cause disease in humans are also found in fruit flies.
Development (tissues and organs)
Genetics and heredity.
Nematode worms
C.elegans: Caenorhabditis elegans.
First multicellular organism genetically sequenced.
Genetics and development.
Little circles on the body are its reproductive tract, they also self-fertilize.
Parkinson’s disease.
Mitochondrial diseases.
The immune system.
Danio rero
Zebra fish:
Vertebrate development.
Toxicology studies.
We share 70% of our genome with this fish.
Cell Culture
Translation studies.
Human cell lines undergo changes to be studied in a petri dish.
Cells undergo differentiation.
HeLa Cells
Cancerous cells.
Genotype
Referring to the genes, all DNA information a cell has.
Phenotype
Appearance, protein expression.
Stem Cells
Can become any cell.
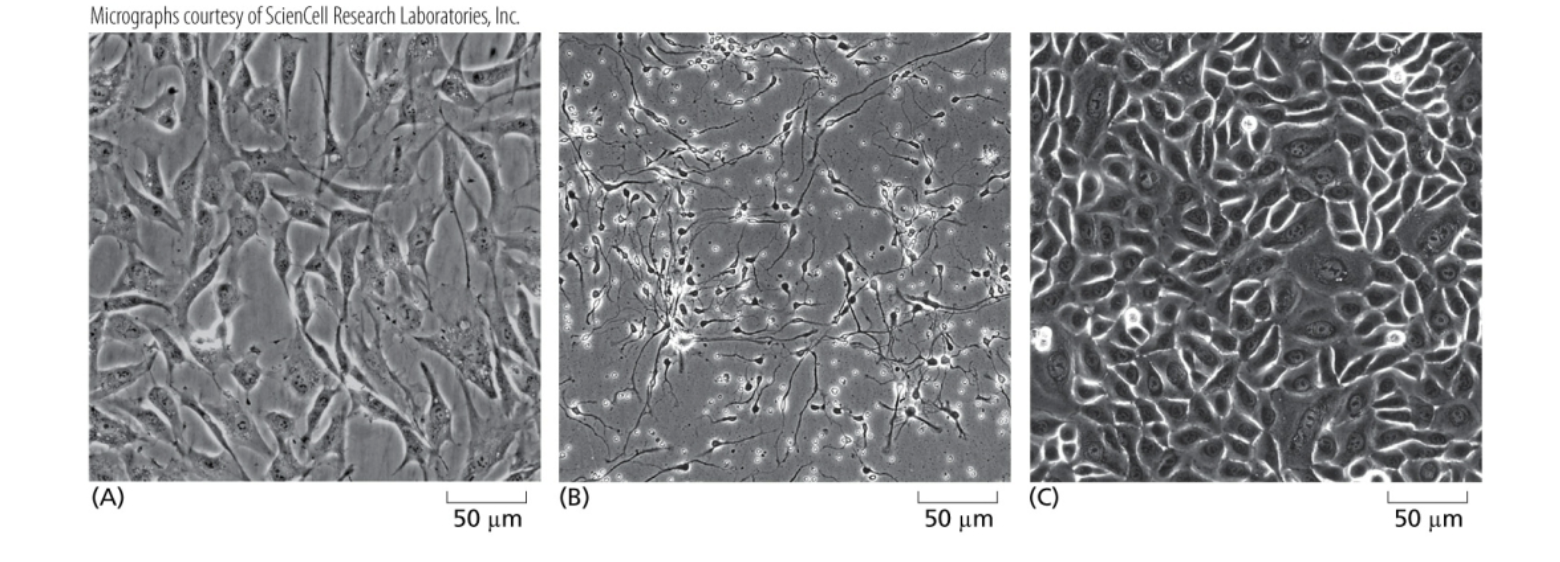
What is the cell types of (A) (B) and (C)?
(A) Fibroblasts from human skin.
(B) Human neurons (thin long process called an axon is visible).
(C) Epithelial cells from human cervix.
Where does an axon grow out of?
The cytoskeleton.
What is Differentiation?
The combination of proteins expressed or produced that gives the cells specific proteins (almost entirely proteins).
Light Microscopy
Makes small structures/samples visible by providing a magnified image of how they interact with visible light.
Uses:
Absorption
Reflection
Scattering
Light moves slower through water than air (moves as a wave).
Using a stain brings out a feature we are interested in.
Where is the objective lens?
Closest to the specimen.
Where is the ocular lens
The eye piece.
Phase Contrast Microscopy
Alters the path of light and creates an artificial change.
Fluorescent Microscopy
Focuses a specific beam onto our sample that will make the cell glow.
Uses dye or molecules to highlight specific features or molecules.
What is DAPI?
Common binding dye.
What is GFP/RFP?
Fluorescent molecules that can be added to track and visualize specific features.
GFP: Green.
RFP: Red.
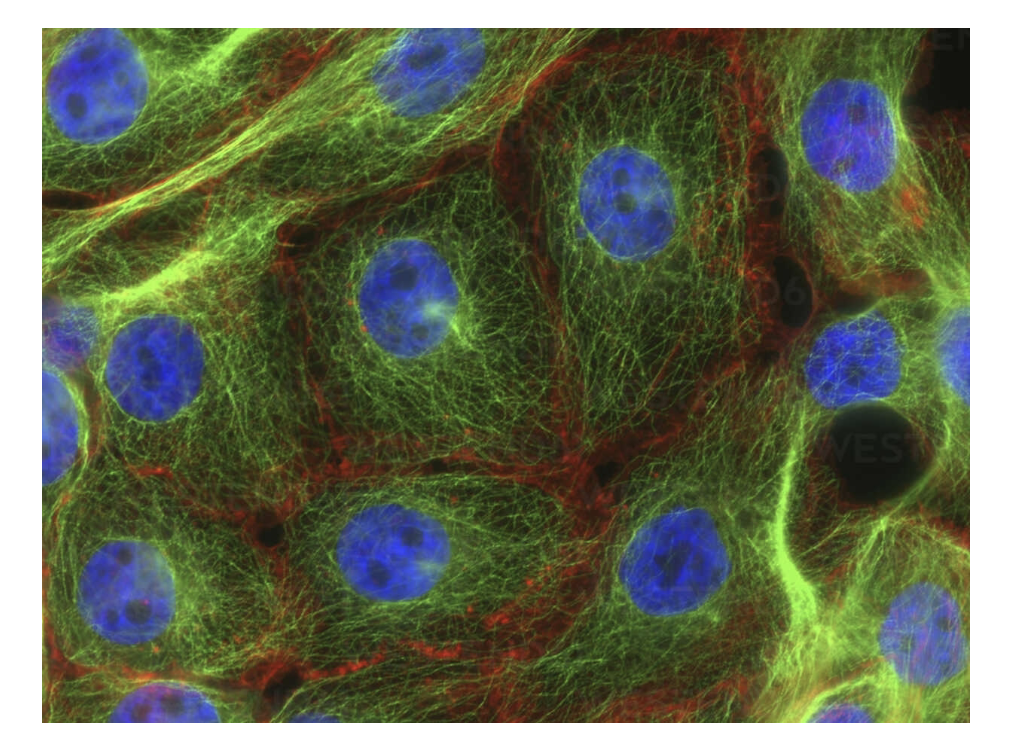
Look at this image of stress fibers in human breast cancer cells and identify DAPI, GFP and RFP
DAPI: DNA binding dye is in blue (nucleus).
GFP: Green (microtubules) pulls chromosomes apart.
RPF: Red around actin (microfilament network)
What is Cryo-Em?
Purifies proteins and then dumps them out to show a structure, then we can lay out the polypeptide chain.
What is the Cell Theory?
All organism are made of cells.
All existing cells are produced by other living cells.
The cell is the most basic unit of life.
Who came up with the Cell Theory?
Matthias Jakob Schleiden and Theodor Schwann.
Why isn’t #2 of the Cell Theory universal?
Evolution had to have happened/a threshold was crossed and a cell came to rise as there was no life on earth when originally.
What is Phagocytosis?
The ingestion of bacteria or other material by specialized cells (amoebas, macrophages, etc).
What did eukaryotic cells most likely originate as?
Predators.
How did mitochondria evolve?
They probably evolved from aerobic bacteria engulfed by an arches-derived, early anaerobic eukaryotic cell and survived inside it, living in symbiosis with their host.
Where are mitochondria and chloroplasts only found in?
Eukaryotes.
How did chloroplasts evolve?
Almost certainly evolved from engulfed photosynthetic bacteria.
What does LUCA stand for?
Last universal common ancestor.
What does LECA stand for?
Last eukaryotic common ancestor.
Is Bacteria or Archaea more closely related to humans?
Archaea.
Is a paramecium a single-celled or multicellular protist?
Single-celled protist.
What are vacuoles?
Storage space that holds substances and nutrients.
How does soft x-ray tomography work?
It slices and rebuilds the segmented organelles, into a 3d structure.
What are protein fusions?
Adds a fluorescent tag (GFP, mRFP) on a protein of interest.
Anywhere the protein of interest goes will be illuminated by the tag.
Almost always doesn’t affect the cells.
What are antibody conjugates?
An antibody with a fluorophore will bind and illuminate a feature.
Almost always doesn’t keep the cells alive.
What is translation?
Nucleic acids to amino acids.
What is transcription?
DNA to RNA.
What do genes provide instructions of?
For the form, function, and behavior of cells and organisms.
What are catalysts and what do they do?
Alter the rate of chemical reactions (specialized proteins, called enzymes, perform these functions).
Manipulate the energetics of chemical reactions to allow life to occur (faster with less energy).
What are the characteristics of prokaryotic cells?
No nucleus or internal membranes.
Have a nucleotide region.
Cluster of nucleotides.
Plasma membrane.
What are the three common shapes of prokaryotic cells?
Spherical (cocci)
Rod-shaped (bacilli)
Spiral
What is cytosol?
A concentrated aqueous gel of large and small molecules.
Regulation of when things can be in proximity of each other.
What 1 distinct element do eukaryotic cells have?
A nucleus.
What is the nuclear envelope?
2 membranes and proteins connected together.
What are nuclear pores?
Regulate what comes in and out of nucleus.
What are nuclear lamins?
Intermediate filaments.
What is the nucleolus?
Special sub-compartment where ribosomes are produced.
What is the cytosol?
(Aq) Viscous medium that makes up the cell.
Mitochondria traits
Oxidative phosphorylation
Binary fission
ATP synthesis
How did mitochondria evolve?
The Archaea cell swallowed it up by phagocytosis, eukaryotes evolved from this.
Chloroplast traits
Capture energy from sunlight and reduce CO2 to generate sugars.
Photosynthesis
Carbon fixation (carbon plus carbohydrates)
What is comparative genomics?
Conserved genetic sequences can yield insight to gene functions across divergent species.
If you have the same protein doing the same thing it must be important what the protein is doing.
What is the 1st gene liked to cancer?
Cdc2 (Cell Division Cycle)
What are genetic screens?
Manipulation of DNA to identify functional interactions.
Often depends on the phenotype analysis.
What are transgenic organisms?
An organism that contains genetic material into which DNA from an unrelated organism has been artificially introduced.
Many different species share similar genes (Ex. Kit gene discoloration of skin (human+mice ex.))
What are molecules composed of?
Atoms that interact with another to form distinct structures that are intimately connected to functions.
Protons
Have a positive charge.
Define which element an atom is.
1 Da
Neutrons
Neutral charge.
Can vary resulting in an isotope.
1 Da.
Electrons
Negative charge.
Undergo reactions to achieve stability, numbers can vary in ions.
1/2000 Da
What are isotopes?
The number of protons + neutrons, informs the isotope identity for a particular element.
Ex. C-12, C-13, and C-14
What is C-14 used for?
Carbon dating as it is unstable and degrades at a predictable rate (1/2 life).
What are biomedical applications?
Radioactive tracers. Ex. F-18 (radioactive isotope)
Electrons go through the electron transport chain to make water when given oxygen.
What is P.E.T
Positron emission tomography scan.
What is a half life (1/2 life)?
A “parent” isotope decays into its “daughter” isotope at a fixed rate.
Varies from seconds to days to years etc.
What is an electron shell?
An electron state of potential energy, each electron shell is subdivided into orbitals.
Consists of a specific number of orbitals( shells are subdivided into orbitals)
Electrons in the first shell has the lowest potential energy. So and increase in shell number increases the potential energy.
What is an orbital?
3 dimensional space where an electron is found 90% of the time, there are 2 electrons per orbital.
What is the valence shell?
The outermost shell.
Atoms in the same vertical column typically gain or lose electrons to attain a filled outer shell.
Atoms want a full valence shell.
Drives stabilizing interactions for atoms.
Elements with a full valence shell are chemically inert (Noble Gases).
What is a molecule?
2 or more atoms held together by chemical bonds.
Ex. O2
What is a compound?
A combination of 2 or more atoms involving different elements held together by chemical bonds.
Ex. H2O
Define and Describe Electronegativity
Electronegativity is the pull that a nucleus has for its electrons.
Upper right on the periodic table is the most electronegative.
Bottom left on the periodic table is the weakest in terms of electronegativity.
What is a covalent bond?
Sharing of electrons.
What is the electronegativity difference for a pure covalent bond?
Less than 0.4.
Electrons are shared equally.
What is the electronegativity difference for a polar covalent bond?
Between 0.4 and 1.8.
Electrons are shared unequally.
What is the electronegativity difference for an ionic bond?
Greater than 1.8.
Electrons are transferred.
What is a polar covalent bond?
Bonds that have unequal sharing that results in partial charges.
What is a partial charge?
1 of the 2 atoms pull the electrons more than the other, while the electrons are spinning they are more likely found at the more electronegative ions (this gives the partial charge).
What is a pure covalent bond?
Bonds that do not have a partial charges.
What is an ionic bond?
Forms between ions (fully charged atoms).
Results in a positive ion (cation) and a negative ion (anion).
Transfer of electrons.
The electrostatic attraction between a cation and anion.
These bonds are weak and break apart in water.
What are intramolecular bonds?
Bonds within the molecule.
What are intermolecular bonds?
Bonds between molecules.
What are hydrogen bonds?
They form by the attraction between positive and negative partial charges.
What are Van der Waals attractions?
Weak attractions that form between non-polar molecules.
Ex. Oil
What is cohesive behavior and surface tension?
Surface tension is a measure of how hard it is to break the surface of a liquid.
Cohesion is the attraction between water molecules contributes to surface tension.
H-bonding between H2O
What is transpiration?
Is the process where plants move water against gravity.
What is adhesion?
An attraction between different molecules.
Ex) H2O and plant cell walls.