Common Molecular Geometries - Unit 2 AP Chem (copy)
1/53
Earn XP
Description and Tags
Common Molecular Geometries in VSEPR theory, with Sigma bonds, Unshared Pairs, and Bond Angles
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
Which molecular geometries have 4 sigma bonds?
Tetrahedral, Square Planar, See-saw
Which molecular geometries have 3 sigma bonds?
Trigonal Pyramidal, Trigonal Planar, T-shaped,
Which molecular geometries have 2 sigma bonds?
Bent, Angular, Linear
Which molecular geometries have 5 sigma bonds?
Square Pyramidal, Trigonal Bipyramidal
Which molecular geometry has 6 sigma bonds?
Octahedral
How many sigma bonds does a Tetrahedral have?
4
How many sigma bonds does a Trigonal Pyramid have?
3
How many sigma bonds does a Bent geometry have?
2
How many sigma bonds does a Trigonal Planar have?
3
How many sigma bonds does an Angular geometry have?
2
How many sigma bonds does an Linear geometry have?
2
How many sigma bonds does an Octahedral have?
6
How many sigma bonds does a Square Pyramidal have?
5
How many sigma bonds does a Square Planar have?
4
How many sigma bonds does a T-shaped geometry have?
3
How many sigma bonds does a Trigonal Bipyramidal have?
5
How many sigma bonds does a See-saw have?
4
How many sigma bonds does a Trigonal Planar have?
3
How many Unshared Pairs does a Tetrahedral have?
0
How many Unshared Pairs does a Trigonal Pyramidal have?
1
How many Unshared Pairs does a Bent geometry have?
2
How many Unshared Pairs does a Trigonal Angular have?
0
How many Unshared Pairs does an Angular Geometry have?
1
How many Unshared Pairs does a Linear geometry have?
0
How many Unshared Pairs does a Octahedral have?
0
How many Unshared Pairs does a Square Pyramidal have?
1
How many Unshared Pairs does a Square Planar have?
2
How many Unshared Pairs does a T-shaped geometry have?
3
How many Unshared Pairs does a Trigonal Bipyramidal have?
0
How many Unshared Pairs does a See-saw geometry have?
1
How many Unshared Pairs does a Trigonal Planar have?
2
What is the bond angle of a Tetrahedral?
109.5 degrees
What is the bond angle of a Trigonal Pyramidal?
107 degrees
What is the bond angle of a Bent geometry?
105 degrees
What is the bond angle of a Trigonal Planar?
120 degrees
What is the bond angle of an Angular geometry?
117 degrees
What is the bond angle of a Linear geometry?
180 degrees
What is the bond angle of an Octohedral?
90 degrees
What is the bond angle of a Square Pyramidal?
90 degrees
What is the bond angle of a Square Planar?
90 degrees
What is the bond angle of a T-shaped geometry?
90 degrees
What is the bond angle of a Trigonal Bipyramidal?
90 degrees and 120 degrees
What is the bond angle of a See-saw geometry?
87 degrees and 117 degrees
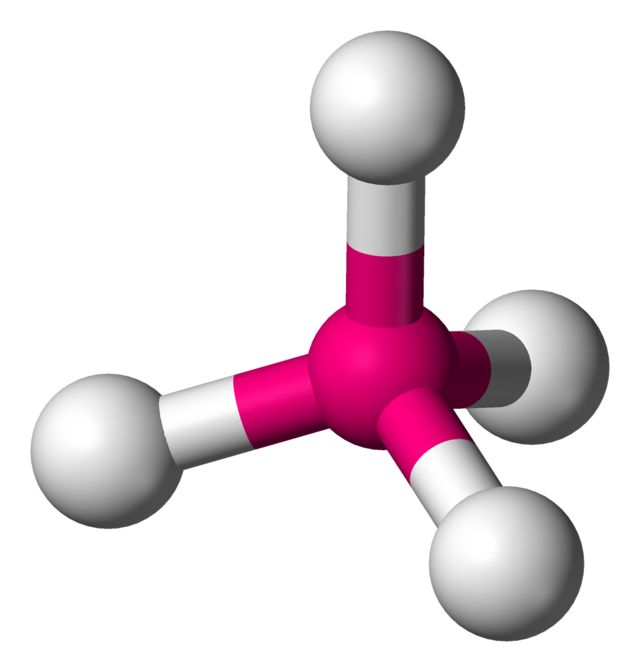
What is this geometrical molecule?
Tetrahedral
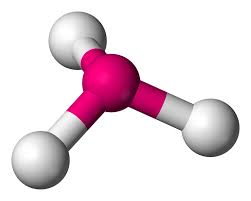
What is this geometrical molecule?
Trigonal Pyramidal
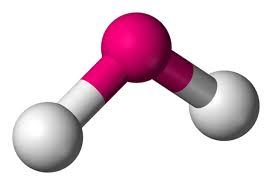
What is this geometrical molecule?
Bent
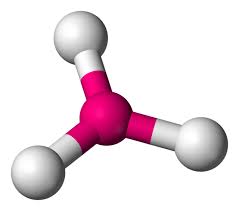
What is this geometrical molecule?
Trigonal Planar
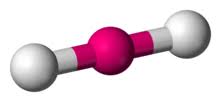
What is this geometrical molecule?
Linear
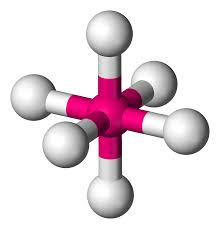
What is this geometrical molecule?
Octohedral
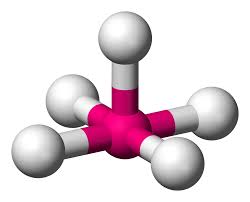
What is this geometrical molecule?
Square pyramidal
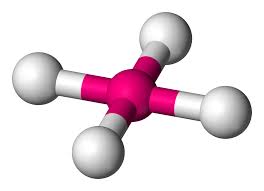
What is this geometrical molecule?
Square planar
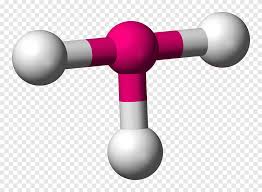
What is this geometrical molecule?
T-shaped
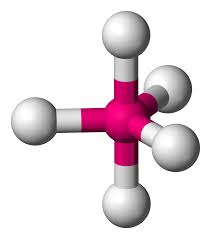
What is this geometrical molecule?
Trigonal Bipyramidal
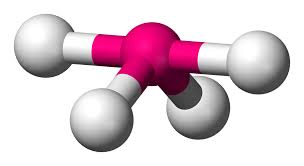
What is this geometrical molecule?
See-saw