Nuclear fission and fusion
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
What is the mass deficit?
The difference in mass before and after a nuclear reaction
Why does the mass deficit happen?
Mass is converted into energy
E=mc^2
What is the mass deficit equivalent to?
The energy released during fission and fusion
Nuclear binding energy
What is binding energy?
The energy needed to separate all of the nucleons in a nucleus (in MeV)
How can we compare binding energies between different nuclear reactions?
By finding the binding energy per nucleon
Binding energy/ number of nucleon
What type of graph would you get if you plot binding energy per nucleon against nucleon number?
A curve
The point of max is where the most energy is needed to remove nucleons apart
It is the most stable here = 56 (iron)
When there are fewer nuclei the force is smaller as there are less forces acting on each particle
As the number of nuclei increases more forces are acting on it causing the binding energy to increase
It does so until the point where it is so large the nuclei at opposite ends experience very week forces
The opposing electrostatic force then takes over as the main force (it decreases at a slower rate)
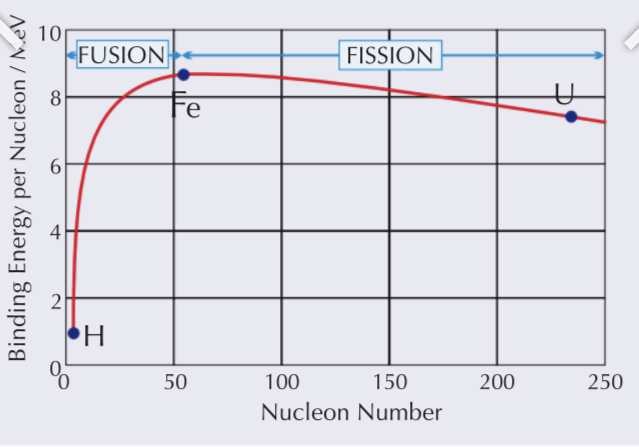
What is fusion?
Combining nuclei
Increases binding energy per nucleon
A lot of energy is released
What is fission?
The splitting of nuclei Into small nuclei
Increases binding energy per nucleon
(Less energy released than for fusion)
How can we estimate the energy released from nuclear reactions?
using the binding energy per nucleon graph
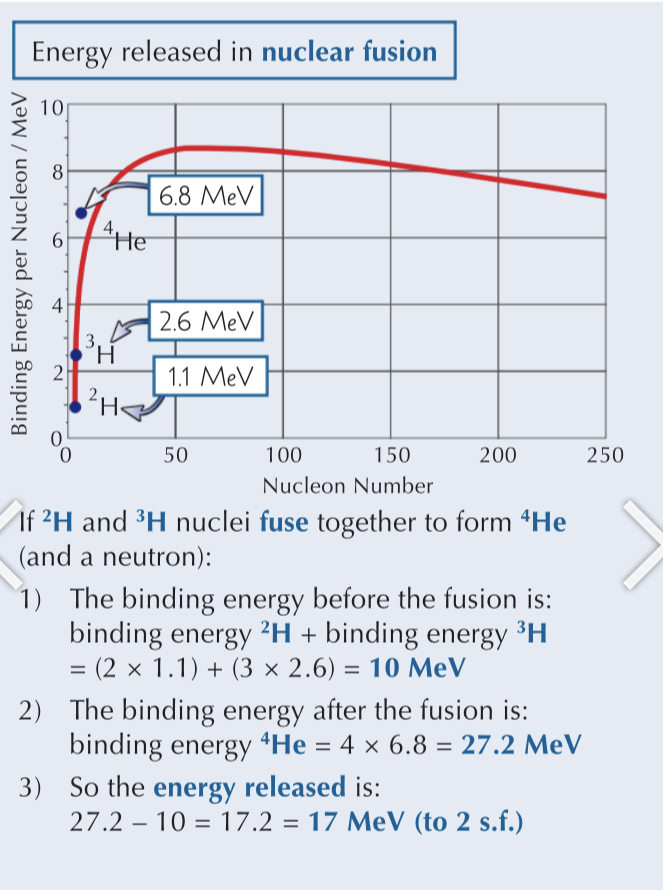
Why does fission occur?
Nuclei are heavy and unstable
They split into lighter nuclei
Energy is released as the nuclei now have a high binding energy per nucleon as the size as decreased (on the right hand side of the graph)
What does nuclear fission limit?
the number of nucleons in a nucleus
the number of possible elements
What are the requirements of fusion?
High temperature and density/pressure
All the nuclei are positively charged so there is an electrostatic force of repulsion between them
Nuclei need to overcome this force to fuse
Pressure needs to be great enough to push them close to each other
They need a lot of KE so requires high temperatures
Fusion in stars
Energy from sun = energy from fusion reactions
Fusion is able to occur as there are very high temperatures and pressures in the sun
Sun has a very high binding energy per nucleon so a lot of energy is released
This maintains the temperature required for fusion
What is plasma?
No atoms uses nuclei as electrons are stripped away
Electrons are free floating