Heat treatment of plain carbon steel
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
Heat treatment
Material is heat treated to
relieve stresses
increase softness, ductility, and toughness
produce a specific microstructure

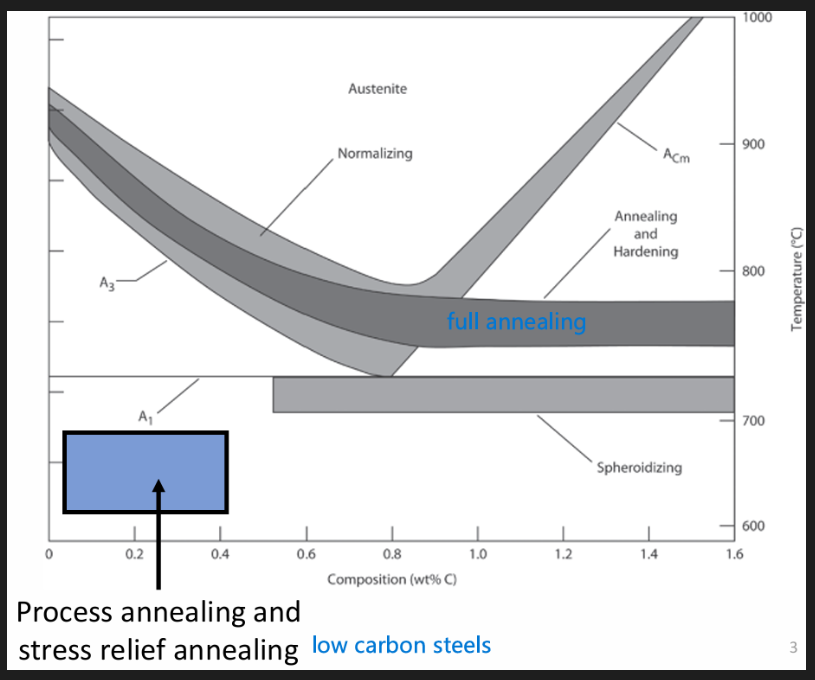
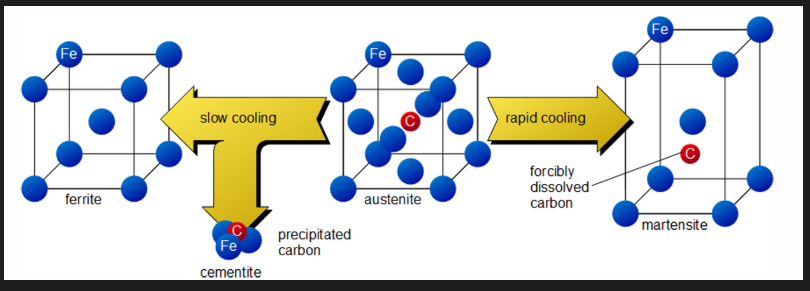
Types of annealing (depends on composition)
Process annealing
Stress relief annealing
Full anneal
Spheroidising anneal
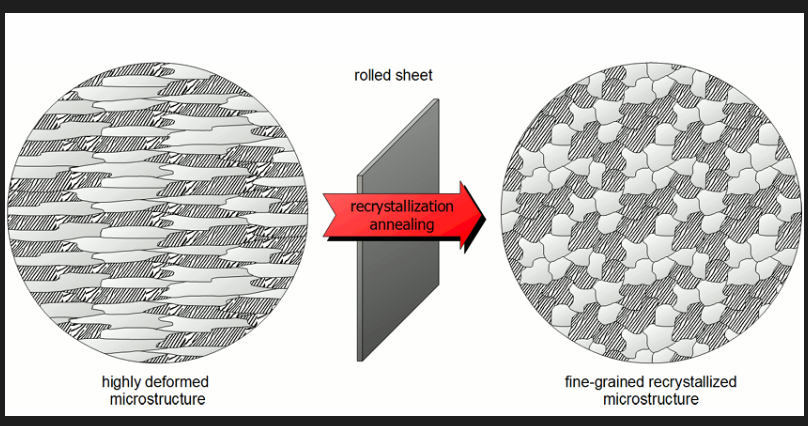
Process annealing
Process annealing
Used for steels below 0.4% to negate affect of CW, for low C steels.
Used during extensive CW to allow continuation of CW without breaking. Recovery and recrystallisation are allowed to occur.
Temp. 630 to 700
Component is allowed to cool in furnace
a fine grain structure is desired
Stress relief annealing
A steel heated or cooled quickly or unevenly can form residual stresses ex: welding, machining, quench hardening. The strength of the workpiece is reduced and distortion can occur.
Internal stresses may be removed by stress relief annealing heat treatment. Part is heated between 550 and 650 degrees. Relatively low such that affects from CW or other heat treatments are not affected. Slowly cooled ex: in switched off furnace or air cooled.
Full annealing
Solidification of steels occurs at temps. much higher than heat treatment process temps. Production of large components ex: casting or forging can result in very large austenite grain sizes. On air cooling, ferrite forms along the austenite grain boundaries and also within the grains to produce a mesh like structure (Windmanstatten). The latter impacts negatively the toughness of steel.
Steel is heated to above A3 (hypoeutectoid) or A1 (hypereutectoid) and allowed to fully form austenite/austenite-cementite grain structure.
Material transformed into the equilibrium microstructure as it is allowed to cool very slow (furnace cooling for alloy steel). Microstructure is coarse pearlite (in addition to any proeutectoid phase). Microstructure with uniform small grains result. Material will get increased ductility, relieved internal stresses, lower hardness and refined grain size and suitable for undergoing other heat treatment processes.
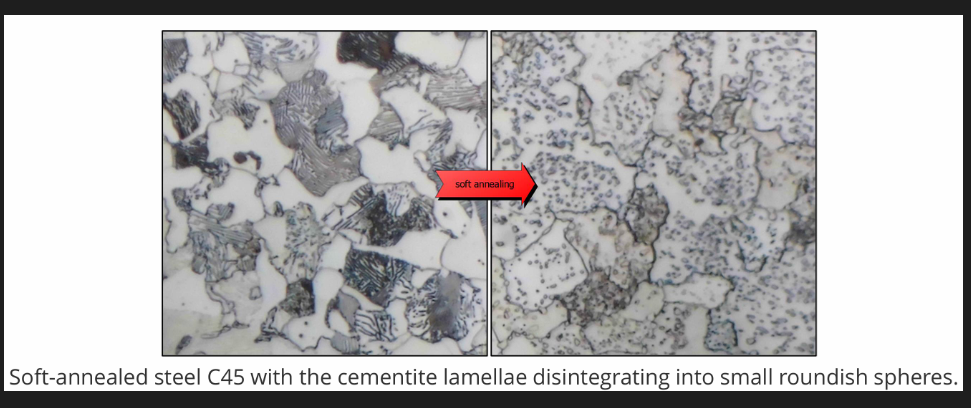
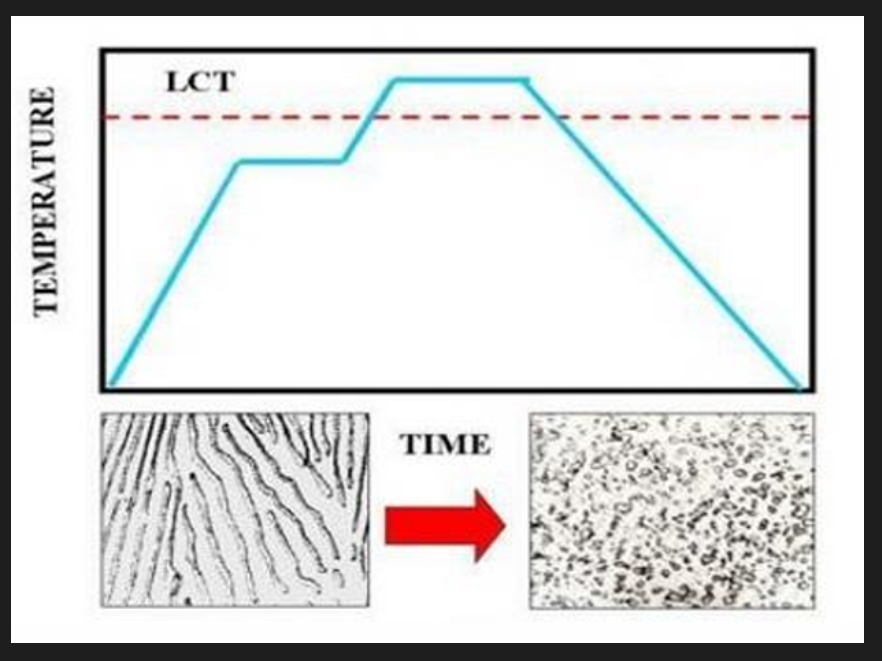
Spheroidising (soft) anneal
Most effective for steels above 0.4%C (significant amount of pearlite). Steel is heated, soaked and cooled slowly to produce spheroidal pearlite/ globular forms of carbides. Generally heat below lower critical temps. but may also involve cycling above and below lower critical temps (+-50) or above lower critical temps. (steel alloys) followed by slow cooling. Reduced hardness; maximises ductility and machinability. For low C %(less than 0.4%) this is not needed as the steels are already quite soft.
This improves the machinability and formability of the steel ex: sheets. Ductility of these steels is higher as dislocation movement is less hindered by spheroidal cementite compared to lamellar pearlite. Spheriodite is easier to machine via continuous cutting operations.
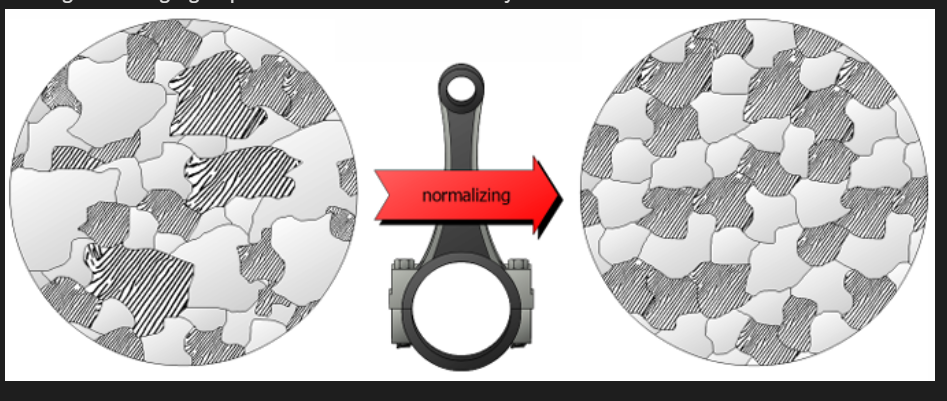
Normalising
Steels which have been plastically deformed consist of grains of pearlite (and a proeutectoid phase) which are irregular in shape and relatively large but vary substantially in size.
During normalising, the steel is heated to above A3 or Acm and allowed to turn into fine austenite. Steel is then cooled freely in air (or a protective gas). Cooling in air, faster.
For hypoeutectoid steels, the fine grains of austenite will then transform into fine ferrite and pearlite on cooling, producing a uniform and desirable distribution of fine grained structure which is tougher than coarse grained.
For hypereutectoid steels, the material is heated above the Acm line to transform to austenite and then cooled to transform into fine pearlite and cementite contributing to a higher strength. Sometimes used to stress relief between rough and fine finishing of large castings and forgings, provides dimensional stability.
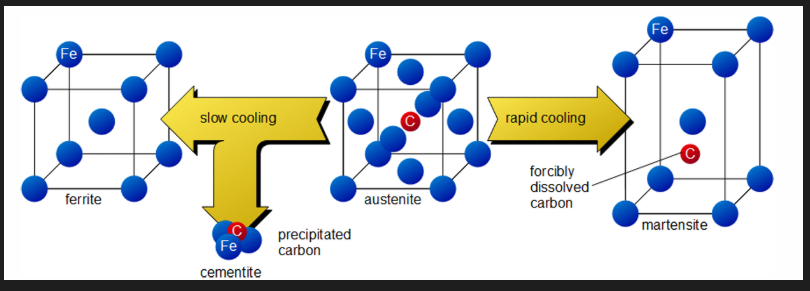
Quench hardening
Temperature band for quench hardening is the same as for full annealing. Amounts lower than 0.4% C do not exhibit appreciable hardening.
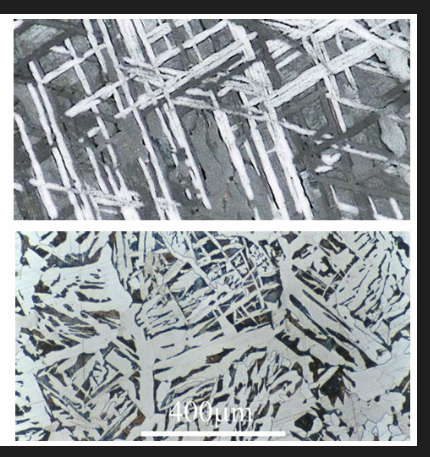
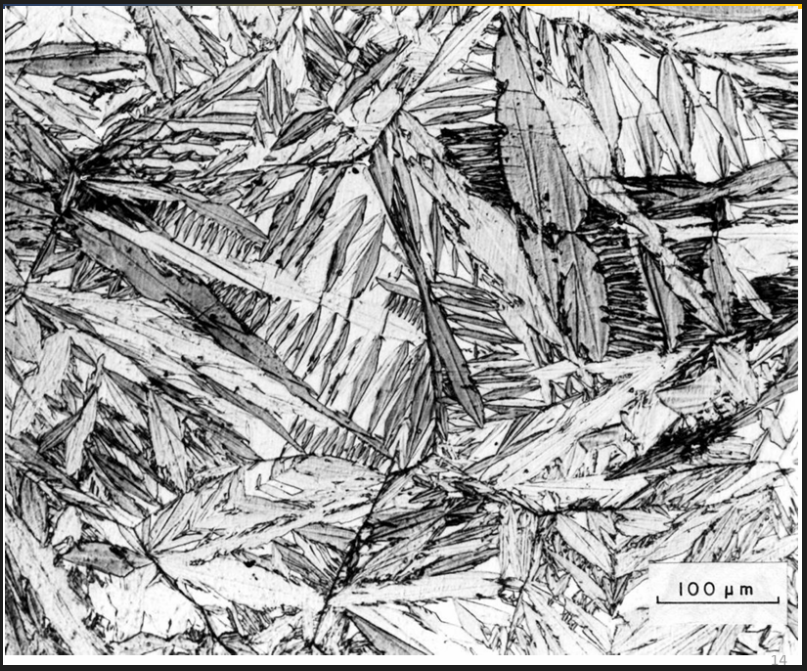
Steel becomes harder when quenched as the C remains in the ferrite and distorts the structure (martensite)
Final hardness depends on the C content and the cooling rate. Martensite is made of needle shaped crystals. C distorts the lattice. Very hard and brittle. Dislocation movement is obstructed (high strength, low ductility)
Limiting ruling section
Max. diameter of a bar cross section which can achieve the required mechanical properties after heat treatment.
Critical cooling rate
Slowest cooling (quenching) rate to produce a martensitic structure throughout the mass of steel.
If CCR is not obtained: steel in centre will cool more slowly, retaining some pearlite instead of martensite. Steel will be tougher but less hard.
If CCR is exceeded: Once max. hardness is reached, increasing the CCR will make the material more prone to cracking and distortion (increased residual stress)
CCR be reduced and any benefits: Reduced by addition of alloying elements enabling thicker components to be hardened with decreased chance of cracking and distortion.
Plain C steels have a high CCR therefore a poor hardenability. Alloys steels with just 3% Ni and 0.3 % C has a low CCR and will harden uniformly. Good hardenability.
Quenching media
Most common quenching media in order of least severe to most severe:
Compressed air blast
Oil
Water
Brine (10% solution)
Choice of quenching bath depends on type of steel and the resultant properties required:
Brine: Simple shapes of plain C steel
Plain water and oils: Plain C steels and alloy steels
Air blasting: High alloy steels and components of small section
Care must be taken to ensure that distortion is kept to a minimum upon quenching (ex: dip long thin components vertically)
Causes of cracking and distortion
Improper steel selection
Improper part design
Inadequate stock removal
Overheating
Improper quenchant selection
Improper fixturing and entry of the part into the quenchant
Long lag times between quenching and tempering
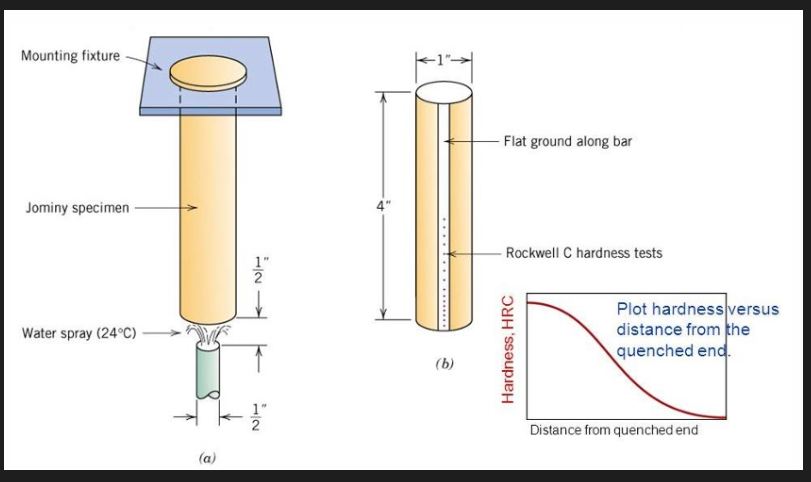
Hardenability
Measure of the steel’s ability to harden by transforming into martensite under set conditions (i.e how easy or difficult it is to harden the steel)
Hardenability is measured using the jominy end quench test. A standardised bar, 25.4 mm diameter and 102 mm long, is heated to the austenitising temperature and then placed on a rig in which one end of the rod is quenched by a standard jet of water.
Measuring: see photo right
What affects hardenability
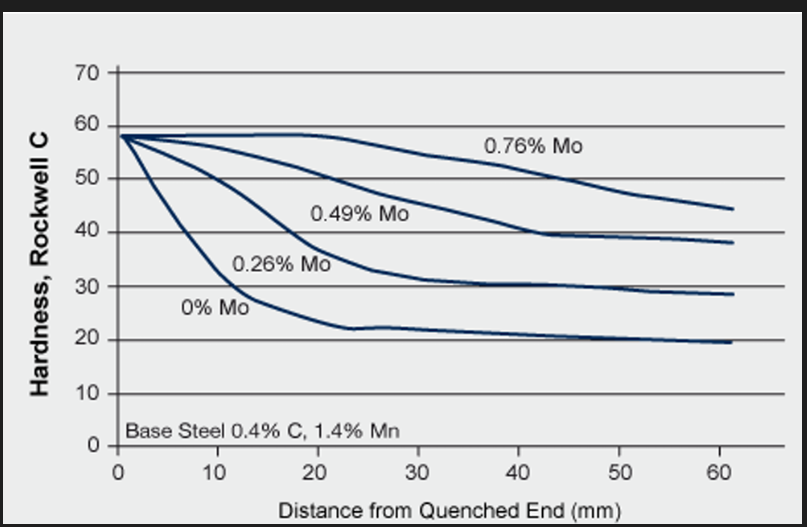
Grain size: In a homogeneous steel, pearlite will nucleate at the austenite grain boundaries. Increasing grain size by increasing the austenitising temp. will increase hardenability (less grain boundaries, less chance of pearlite forming). Ductility and toughness are lower with larger grains. Addition of alloying elements is a better way to increase hardenability.
Alloying elements: 3 mains groups are used:
Carbon: Most important element. Its hardness increases with %C to a max. of around 63 HRC at 0.7% C. Above 0.7%, Mstart line is reduced to below room temp. and therefore the structure does not turn fully martensitic. Limit to C. Big effect on toughness, ductility, joinability and machinability as they are all reduced. Hardenability is increased but not usually used for hardenability as the other properties are affected negatively.
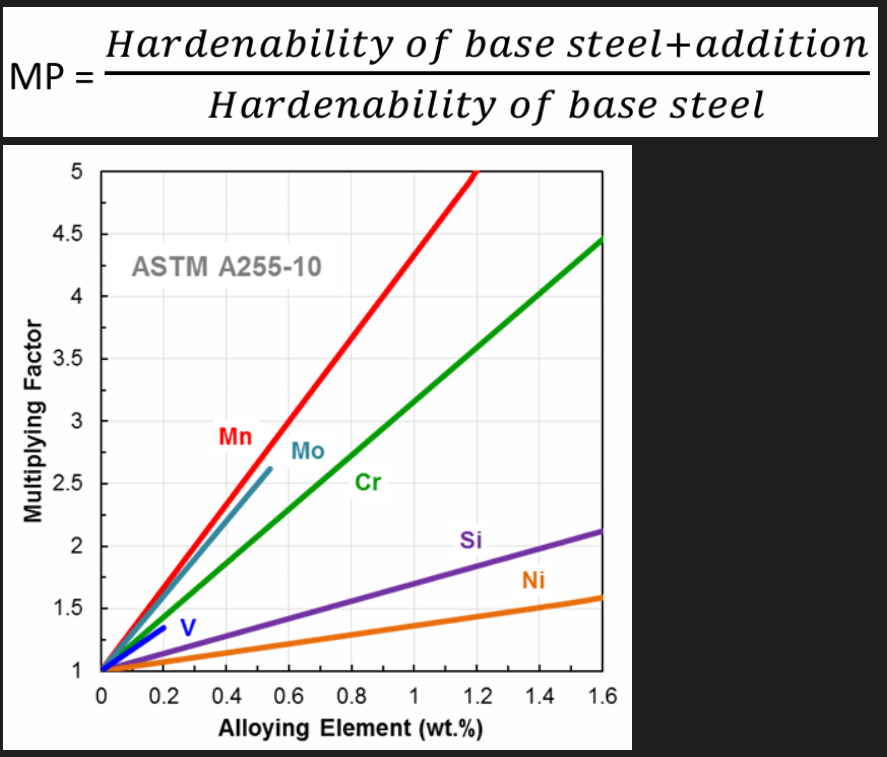
General alloying elements (Cr, Mn, Mo, Si, V, W and Ni): All alloying elements except Co after the austenite transformation and will influence the hardenability . How efficient the elements are in doing this depends on the multiplying MP. Delays the austenite transformation to the equilibrium phases and increase the hardenability. The better the elements are at being hardenability agents, the steeper the line.
Boron: 0.002 to 0.003% B (small additions) produce a very big hardenability potential equivalent to addition of 0.5% Mo, 0.7% Cr, 1% Ni. The hardenability effect is constant as long as minimum level of B is present. The effectiveness is high for low C steel and moves ineffectively towards 0 at the eutectoid composition.
Boron reacts with oxygen and nitrogen therefore it needs to be added with other additives. These additives react with oxygen or nitrogen instead of boron leaving it in a soluble form (e.g. Al or Ti). Boron will segregate at the austenite grain boundaries and suppress transformations at high temperatures to ferrite and pearlite increasing hardenability.
Boron steel: Obtain high yield strength and high toughness. Low carbon steels with boron respond well to hardening and can be cold formed (good for high strength fasteners and nuts/bolts).
Other applications: Vehicles (thinner cross sections therefore lighter), fork lift truck arms and agricultural/lawnmower blades.
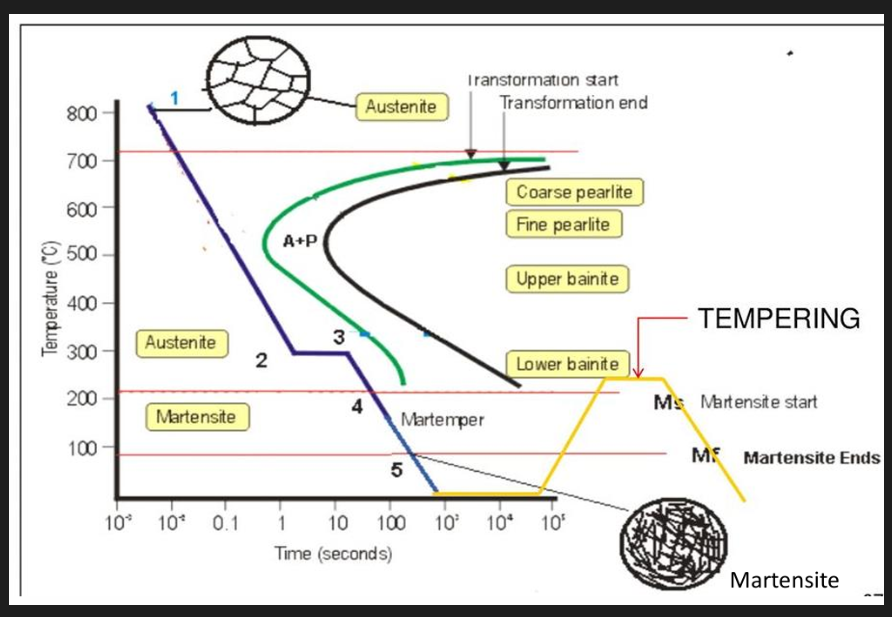
Tempering
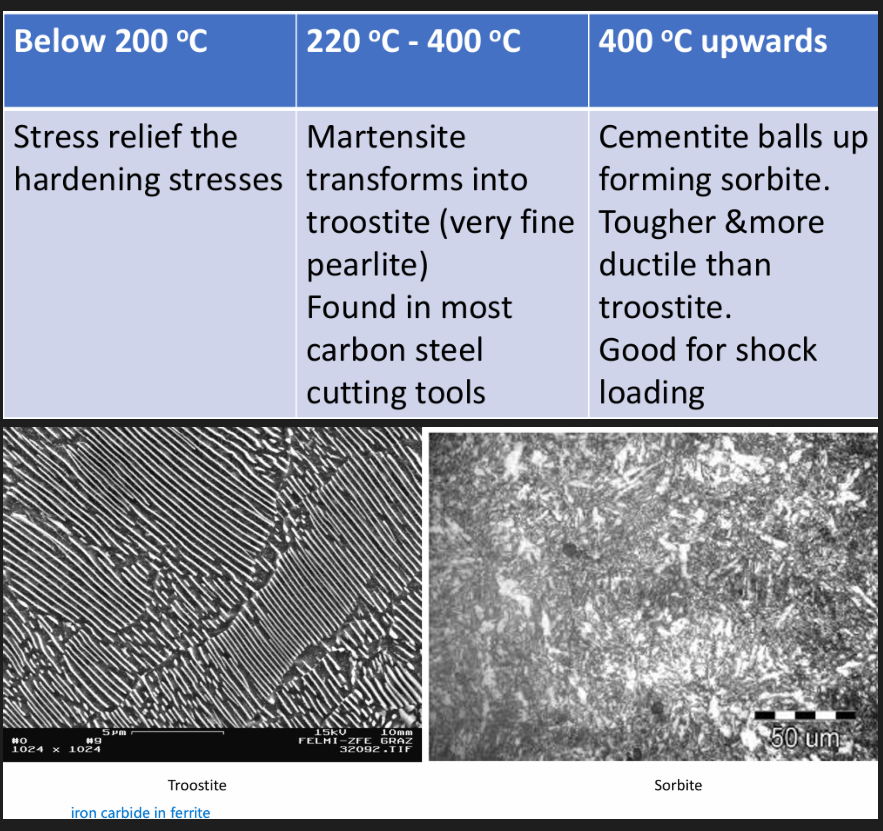
After quenching, the steel will be brittle (due to martensite). The steel is reheated to relieve some stresses and reduce the brittleness by transforming martensite. Compilation of strength and toughness.
Toughness of material is increased. Drop in hardness with tempering temp., transforming microstructure into pearlite and carbide.
Steel softens. Decreased strength, increased ductility. Dislocation density reduced and cementite coarsened. Dislocation density reduced and cementite coarsened. Softening during tempering can be reduced by adding Mo, Cr, V, Nb and Ti. Sufficient amount of carbide forming elements can produce hardness increase at higher temperatures (secondary hardening).
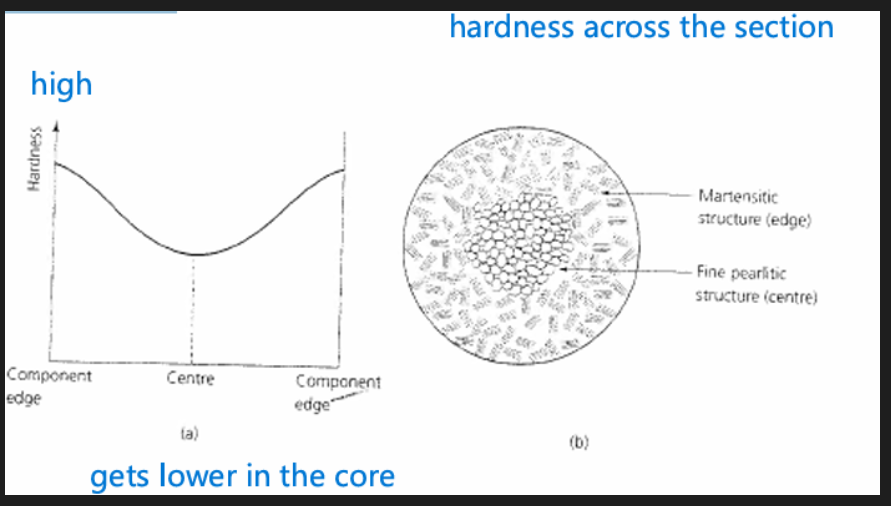
Mass effect
For a thick component, heat gets trapped in the middle, core cools much more slowly. Centre will have a different hardness than edges. Material at edge is cooling much quicker than the material at the centre.
Martempering
Developed to reduce residual stresses during quenching. Steel quenched from austenitising temperature in a hot fluid medium (ex hot oil, molten salt) held at a temperature above Ms line. Held until temperature in steel component temperature is substantially uniform throughout and then cooled at a moderate rate. Steel is then tempered (heated again to reduce brittleness) Advantage over rapid cooling: thermal homogenisation lower residual stress, distortion and cracking.