AP Chemistry Ultimate Guide (copy)
Unit One: Atomic Structure and Properties
The Periodic Table
The periodic table gives you very basic but essential information about each element, take carbon for example:
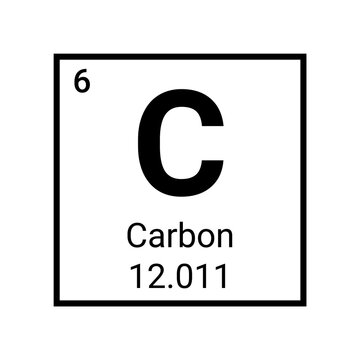
Pictured above is the element carbon as displayed on the periodic table
The symbol (C) tells you the name: carbon. C and carbon can be used interchangeably
The atomic number (6) tells you how many protons and neutrons in the element in addition to the amount of electrons surrounding the element when it is neutrally charged
The molar mass (12.011) tells you two things:
The average atomic mass of a single atom of carbon, measured in atomic mass units (amus)
The average mass of a mole of carbon atoms, measured in grams. 12.011 is how many grams of carbon atoms there are in a mole.
Horizontal rows of the periodic table are called periods
Vertical columns are called groups
Groups can be numbered in Roman numerals or simply as 1-18, but some are also named
Group IA/1-- Alkali Metals
Group IIA/2 -- Alkaline Earth Metals
Group B/3-12 -- Transition Metals
Group VIIA/17 -- Halogens
Group VIIIA/18 -- Noble Gases
There is also two rows beneath the periodic table, the lanthanides and actinides, the rare Earth elements, or inner transition metals.
The identity of an atom is determined by the amount of protons in the nucleus, which also contains neutrons. The mass number of atom is the sum of its neutrons and protons (valued at 1 each). Electrons have significantly less mass than protons and neutrons and do not contribute to the mass.
Isotopes are atoms of an element with different numbers of neutrons, but same amount of protons. For example, carbon-12 has 6 protons and 6 neutrons, but carbon-14 has 6 protons and 8 neutrons. The molar mass listed on the periodic table is determined by the average of the mass numbers of all known isotopes of an element weighted by their percent abundance.
The mass of various isotopes of an element can be determined by a technique called mass spectrometry. Below is a mass spectrum for selenium
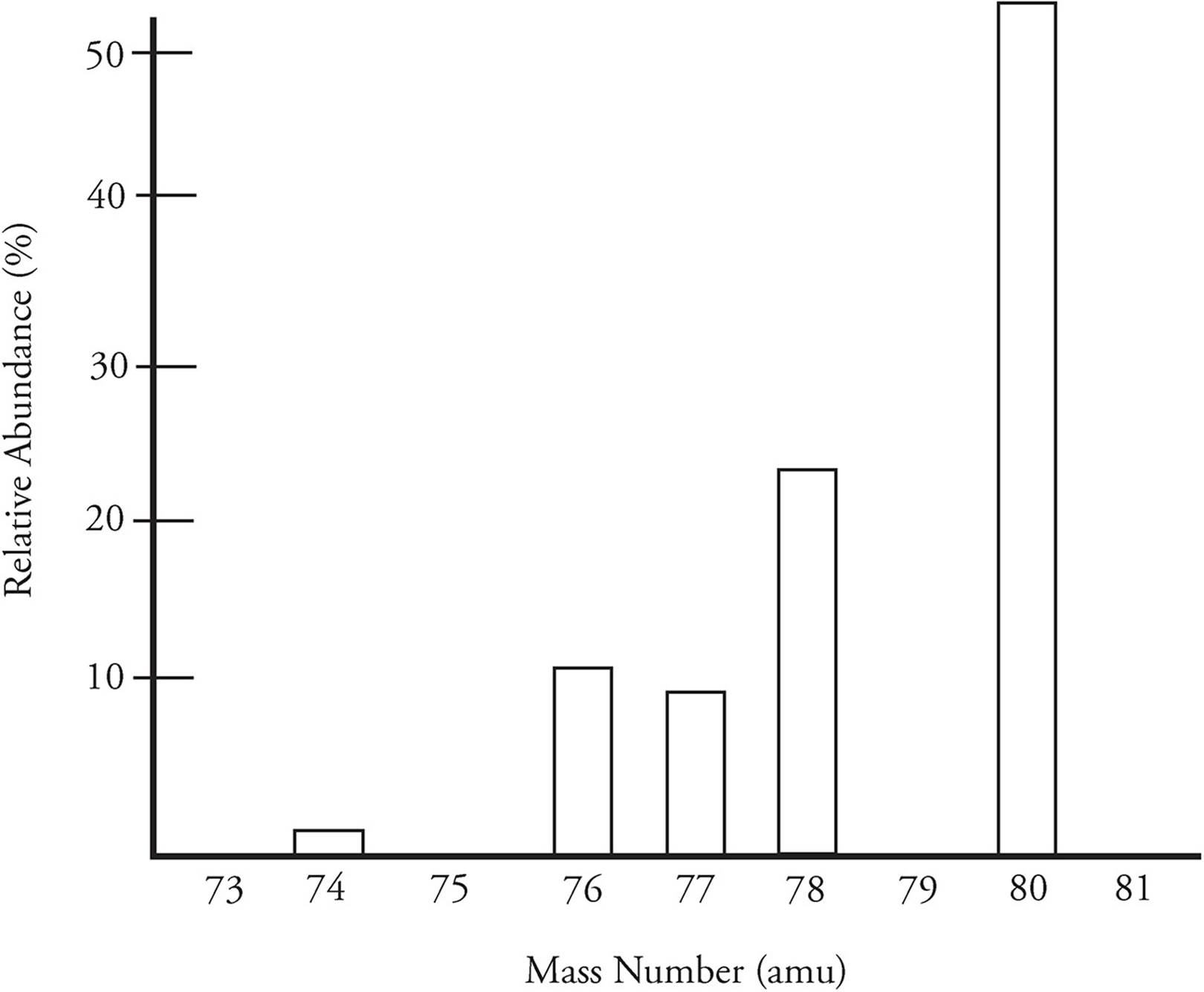
The most abundant isotope of selenium has a mass of 80, but there are four other naturally occurring isotopes. The five isotopes are combined to created a weighted average, which is then the average atomic mass of selenium.
The molar mass of an element will give you a general idea of the most common isotope. Carbon’s molar mass is 12.01, and 99 percent of all carbon in existence is carbon-12.
Moles
The quantity ‘mole’ connects all of the different quantities in chemical equations. Coefficients in chemical equations tells you how many moles there are of that substance.
Moles and Molecules
Avogadro’s Number is the number of atoms in a single mole of any given element. Similar to how a dozen is always 12, Avogadro’s Number is always 6.022x10^23.
1 mole = 6.022x10^23 particles
Moles = particles/(6.022x10^23)
Moles and Grams
You can convert from moles to grams and vice versa by looking at an elements atomic mass. Atomic mass is given in atomic mass units (Amu), which also signifies grams.
Example: one carbon atom has a mass of 12.011 amu, therefore, one mol of Carbon atoms has a mass of 12.011 grams.
Moles = grams/ molar mass
Moles and Gases
When you know the properties of a gas, you can use the ideal gas law equation to solve for moles.
PV=nRT where P = pressure in atm, V = volume (liters), n = mol, R = .0821 (ideal gas constant), T = temperature (kelvin).
However, many gas equations take place at STP, or standard temperature and pressure, where P =1 atm, T=273 Kelvin. at STP converting is easier because one mol ALWAYS occupies 22.4 liters.
Moles = liters/(22.4 L/ mol)
Molarity
Molarity (M) expresses the concentration of a solution in terms of volume. Molarity is used in equilibrium calculations, acids and bases, electrochemistry, and more. When an element is shown in brackets, it's being referred to in terms of Molarity.
[Na+] means "the molar concentration of sodium ions".
Percent Composition
Percent composition is the percent by mass of each element in a compound. To do this, you must divide the mass of each element or component in the compound by the total mass of the compound.
Example: calculate the percent composition each element in calcium nitrate Ca(NO3)2.
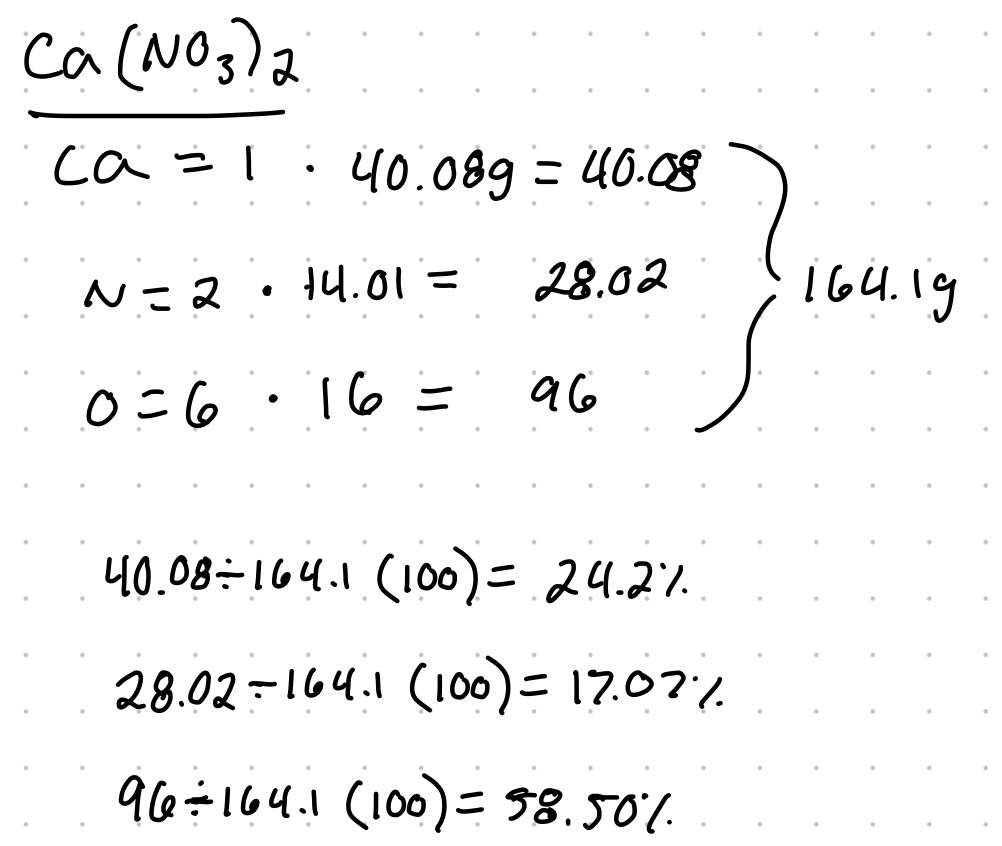
You can add up the percentages to check your work.
Empirical and Molecular Formulas
When given masses or percent mass, you need to be able to convert that to empirical and/or molecular formulas. The empirical formula is the simplest ratio of one element in a compound to another (CH2O). The molecular formula represents the actual formula for the substrate (C6H12O6).
Example: A compound is found to contain 56.5% carbon, 7.11% hydrogen, and 36.4% phosphorus. Part A: determine the empirical formula for the compound.

Part b: if the molar mass is 170.14 g/mol, what's the molecular formula?
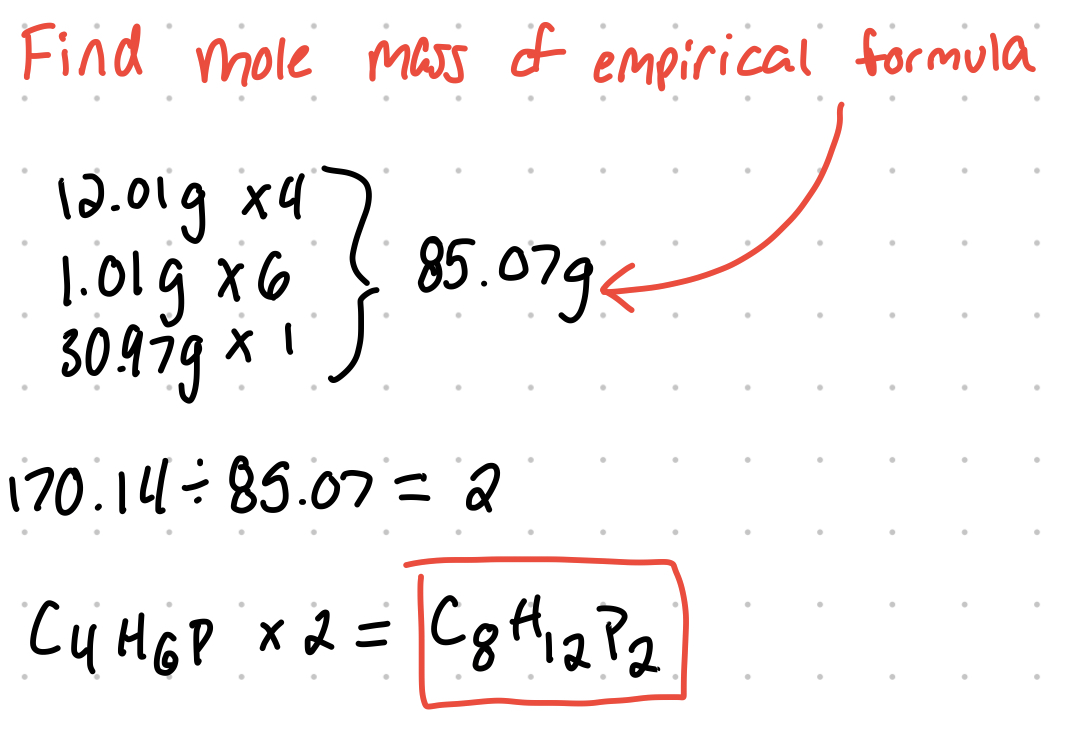
Electron Configurations and the Periodic Table
The positively charged nucleus is constantly attracting the negatively charged electrons. The farther an electron is from the nucleus, the more the potential energy is.
The energy of electrons is quantized, which means electrons can only exist at certain energy levels, separated by specific intervals.
Coulomb's Law
The electrostatic force is the attraction between opposite charges and the strength can vary depending on now for the charges are from each other. To find the strength, you can use Coulomb's law
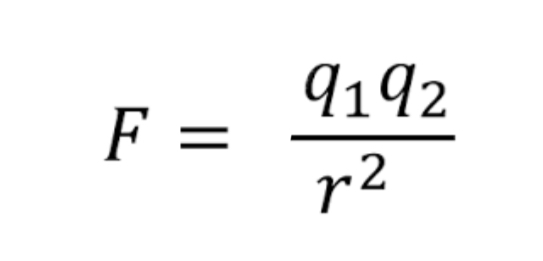
The image of shows the formula for coulomb's law, where F= electrostatic force between nucleus and electron, q1 = magnitude of the positive charge, q2 = magnitude of the negative charge, and r= distance between charges.
Essentially, Coulomb's law states the closer an electron is to the nucleus, the stronger the attraction and the less potential energy there is. in order to remove an electron from an atom, you must add enough energy to overcome the electrostatic force (the binding energy which is always a positive value).
The Bohr Model
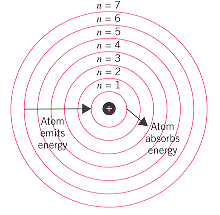
Each energy level correlates to a row on the periodic table. The closer an energy level is to the nucleus, the less electrons that can be in that level. The Bohr model serves the purpose of understanding atomic structure rather than structural accuracy.
When electrons absorb electromagnetic radiation, a form of energy, the electrons can jump to higher energy levels. When electrons drop in energy levels, the emit electromagnetic radiation.
Photoelectron Spectroscopy
To eject an electron from an atom, the ionization energy must be reached in order overcome the binding energies of other electrons in the atom. When examining the spectrum of a single atom, or a small amount atoms, it will be measured in electronvolts (EV), where 1EV= 1.6x10^-19 joules. If measuring moles of atoms, use kJ/mol or MJ/mol.
When the incoming radiation energy overcomes the binding energy and ejects the electron and there's still radiation left over, the radiation will be converted into kinetic energy for the ejected electron.
Incoming radiation energy= binding energy + kinetic energy.
More kinetic energy means faster an ejected electron is moving. The farther an electron is from the nucleus, the less energy it takes to eject. How far an electron is from the nucleus can be determined by looking at the speed of the electron.
Spectra
A photoelectron spectrum (PES) is a graph of the ionization energies for all electrons when ejected from the nucleus.
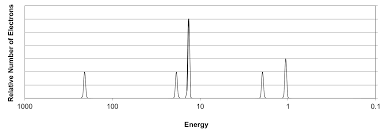
The y-axis, relative number of electrons, is the amount of electrons ejected from a specific energy level.The x-axis shows the binding energy of those electrons which decreases left to right.
Each section of the peaks represents a different energy level and each gap signifies a change in energy level. Because there are multiple peaks in each section, this shows subshells that have different distances. A subshell is the shape of the space an electron can be found in.
The first subshell is the s-subshell that can hold 2 electrons. Second is the p-subshell that can hold six electrons, as shown in the graph by being three times as tall. The height can help determine the amount of electrons in a shell.
In the area for the 3rd energy level, the p-subshell isn't as tall as the other, indicating a partially filled energy level.
Electron Configuration
The periodic table is divided into four subshells, with the size of the atom determining how many subshells it has. Subshells are known as s, p, d (10 max electrons), and f (14 electrons).
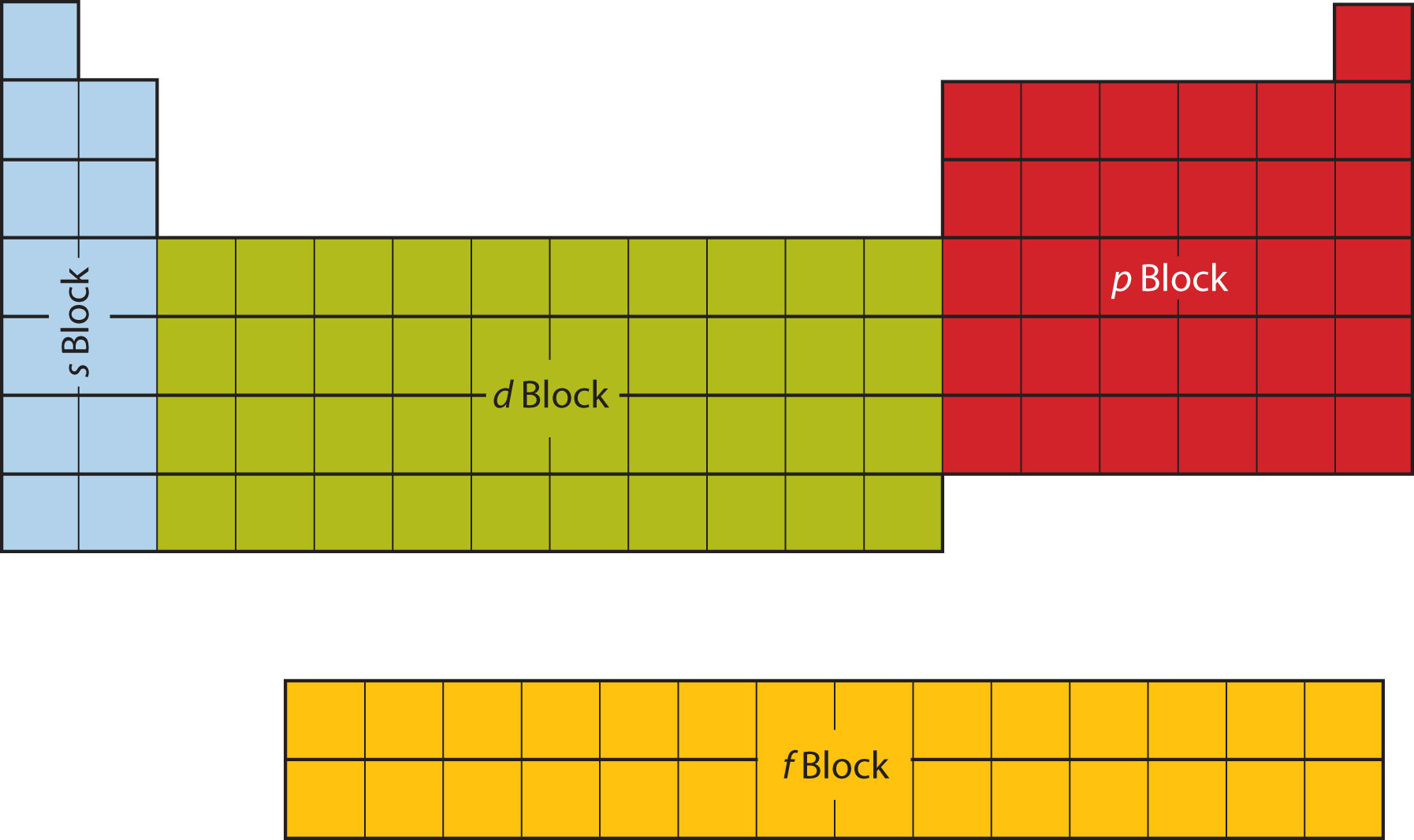
Each subshell is as long as there are electrons in it (s block can have 2 electrons and is two elements long). This method of organization is called electron configuration and can also be used to describe elements.
Configuration Rules
The Aufbau Principle
Aufbau principle says electrons must fill up orbitals, subshells, and shells in order of increasing energy.
The Pauli Exclusion Principle
The Pauli exclusion principle states that when two electrons fill up an orbital, they must spin in opposite directions: clockwise and counterclockwise.
Hund's Rule
According to Hund's Rule, when electrons are filling the orbitals of the subshell, they will only have two in one orbital when it is not possible to have each electron in its own orbital.
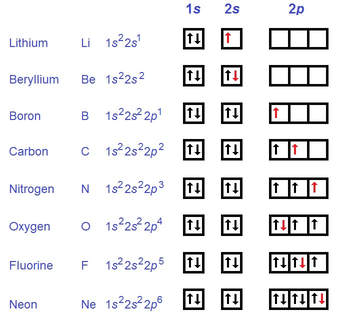
Notice in the above picture howthe arrows are pointing in different directions, signifying different spin, and the arrows will only pair up when there's no other free orbitals (nitrogen and oxygen).
Predicting Ionic Charges
The most stable configuration of an element involves having a completely full outer shell. For any element in the s or p block, that means gaining a total of eight electrons in the outer shell (2 in the s, 6 in the p), known as valence electrons.
When an element gains or loses electrons to become stable they are called ions. when an element is close to a full shell, such as the halogens, they will gain electrons and be negatively charged, these are referred to as anions.
Halogens as ions will be referred to as Cl^- or F^-1
Elements in the oxygen group need 2 electrons for stability, so as ions they have a charge -2 (O^-2)
When elements such as the Alkali Metals want to reach stable configuration, they will lose electrons because it takes less electrons than trying to gain. These are called cations when they are positively charged.
Alkali metals have an ionization charge of +1, and alkaline earth metals have an ionization charge of +2, and so on.

Transition metals are cations, but can have multiple different charges which depends on which compound they are in.
For example, in CuBr2, bromine has a charge of -1 but because there are two bromide ions, that makes their charge -2. in order to have a stable compound, the charge must be zero, which causes the copper charge to be +2. This would be called copper (ii) bromide.
CuBr would be copper (i) bromide.
Only zinc (+2) and silver (+1) are the only transition metals with one charge possibility.
Periodic Trends
There are three basic rules to understanding trends in the periodic table that help define element behaviors:
Electrons are attracted to the protons in the nucleus
The attraction can increase by having a smaller distance between the electron and proton and by having more protons in the nucleus
Electron shielding is when electrons in the inner shells repel electrons in outer shells because they have the same charge, thus decreasing the attraction between the outer electrons and protons
Completed shells are stable and elements will try to lose/gain electrons to complete shells, if possible.
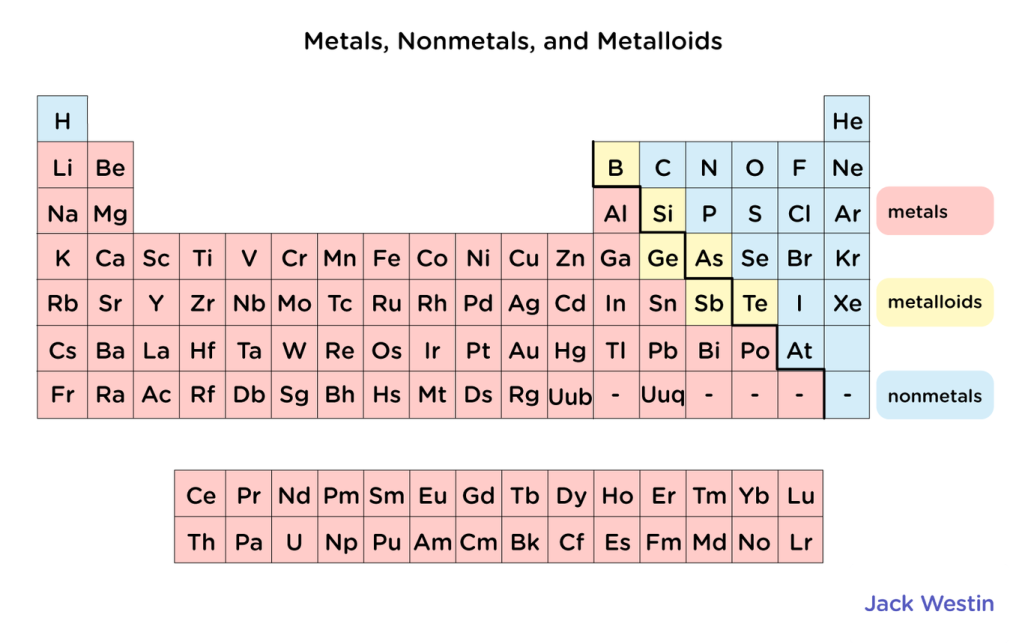
Elements are defined into three categories: metals, metalloid, and nonmetals.
Metals are on the left side of the periodic table, they give up electrons when forming bonds
As the metals go more to the right, the less metallic they behave
Lithium behaves more metallic than zinc.
Nonmetals are on the right side and gain electrons in bonds
Metalloids, which have a metallic and nonmetallic character, separate metals and nonmetals.
Atomic Radius
The approximate distance between the nucleus of the atom to the valence electrons
Moving from left to right, atomic radius decreases. For example, Lithium has a smaller atomic radius than Fluorine. As the atomic number increases, so does the amount of protons and electrons. Because there are more protons in the nucleus and the same amount of electron shells--despite there being more electrons--there is less of an electron shielding effect.
Moving down a group, such as from Lithium to Cesium, the atomic radius increases because even though there’s a stronger positive charge, there are more electron shells that increases the shielding effect and pushes electrons farther away.
Cations are smaller than atoms because electrons are being taken away, electron-electron repulsions are reduced, and there is a stronger positive than negative charge.
Anions are larger than atoms because the atom has an overall negative charge, increasing the amount of electron-electron repulsions.
Ionization Energy
To remove electrons from an atom, it requires energy, called the first ionization energy, and creates a cation. To remove another electron, that’s the second ionization energy, and etc.
Left to right, the ionization energy increases because electrons are closer to the nucleus (protons) and it takes more energy to pull them away from the positive charge.
Ionization energy decreases when moving down a group because there are more electron shells that cause electron shielding and repulsion, causing the electrons to be not as attracted to the nucleus and easily removed.
The second ionization energy is greater than the first because the proton to electron ratio has a stronger favor for protons, this causes the electrons to have a stronger attraction than previous and a higher energy is needed to remove a second electron.
Once a shell is empty, the ionization energy greatly increases because it is closer to the nucleus.
Electronegativity
Electronegativity is how much an element’s nucleus attracts electrons. Two factors contribute to electronegativity: element size (the smaller the more electronegative) and how close an element is to completing a shell.
Left to right, electronegativity increases
Up to down, electronegativity decreases.
Fluorine is the most electronegative element because it is the smallest element that requires only one electron to complete the shell.
Unit Two: Molecular and Ionic Compound Structure and Properties
Bonds Overview
Atoms engage in bonding to share electrons and achieve a stable, lower-energy state. This bonding can either be ionic where an atom gives the electron away, or it can be covalent where they share the electron.
Ionic Bonds
Ionic bonds are between a nonmetal and metal held together in a lattice structure by electrostatic attraction. To bond, the cation gives up the electron(s) entirely to the anion.
The elements are held together by electrostatic force because the positively charged cation is attracted to the negatively charged anion.
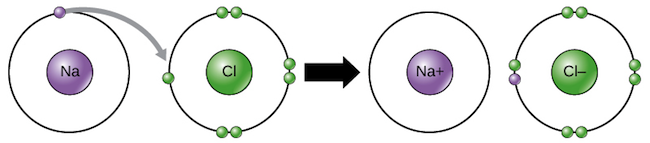
Any substance held together by ionic bonds will be strong, usually solid at room temp, and very high melting and boiling point.
Melting point for ionic substances can vary depending on the charge. Per Coulomb's Law, a greater charge leads to greater bond energy (MgO with a charge of +2/-2 has a stronger bond than NaCl with a charge of +1/-1).
if the charges are the same, then the size is considered. The smaller the ions, the greater the bond energy.
Ionic solids in the lattice structure are poor conductors because the electron is stuck around the anion. Liquids are good conductors because the electrons are free to move despite being locked around one atom. Salts are held together by ionic bonds.
Metallic Bonds
Rather than the electron being stuck around one atom, like in ionic bonds, metallic bonds have a "sea of electrons”. The valence electrons freely move about the bond, making them good conductors, and malleable and ductile.
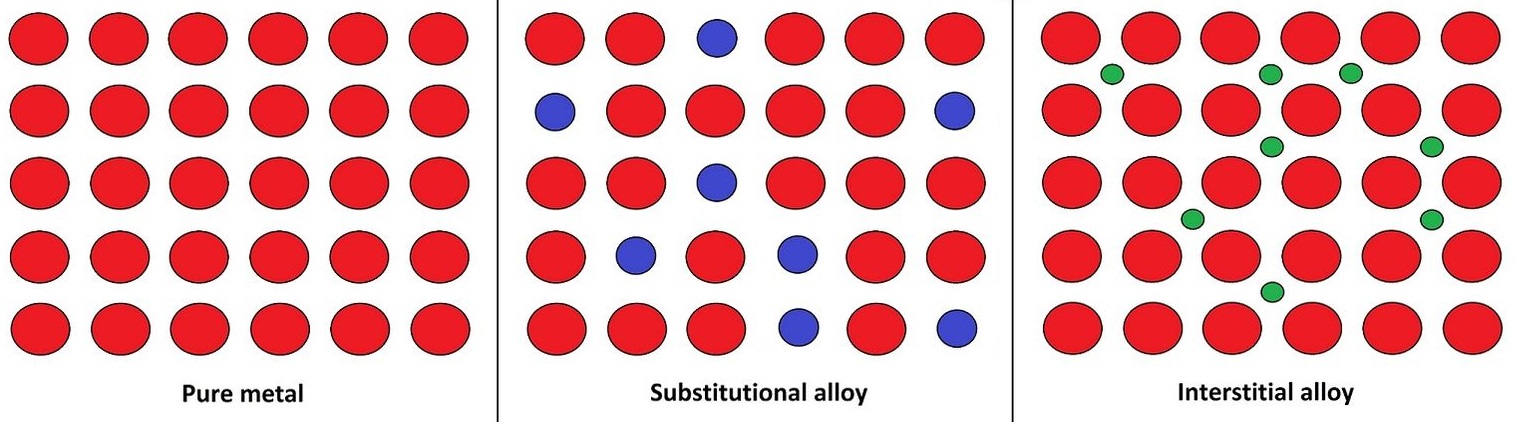
Metals can be made into alloys.
interstitial alloys occur when there are two metals with vastly different radii combine. Steel has large iron atoms and small carbon atoms.
substitutional alloys occur when metals of similar radii combine. Brass has copper and zinc, both similar sizes.
Molecular Covalent Bonding
In covalent bonds, two nonmetals share electrons in the valence shell of the two atoms.
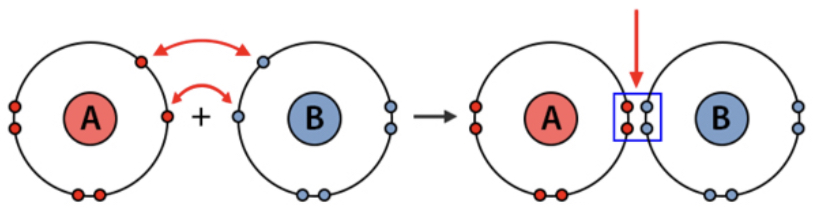
When two or more atoms bond covalently, they create a molecule, which can be as small as two atoms or even as large as 24, there's no size limit.
The first covalent bond between two atoms is called a sigma bond. all single bonds are also sigma bonds. The second bond, a double bond, is called a pi bond. The second and third bond in a triple bond is a pi bond. Double and triple bonds are shorter and stronger than single bonds, but not double or triple the strength,
Internuclear Distance
The length of a covalent bond depends on balancing the attractive and repulsive forces. when two atoms are too close, the potential energy is high, and the nuclei reset each other. When they are too for, the potential energy is zero because they cannot attract.
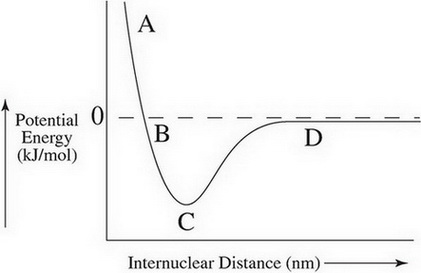
The dashed line represents zero potential energy, but it can be negative, which represents lower energy. at label A, atoms are too close to bond. at label D they are too far. at label C the repulsive and attractive forces are equal and they can bond.
Network Covalent Bonds
Network covalent bonds as solids are held together in a lattice of covalent bonds which makes them very hard, very high melting point and boiling point. Electrons cannot more about the lattice, making them poor conductors.
The most common network solids are carbon (graphite or diamond) and silicon (quartz) because of the four valence electrons that allows more covalent bonding.
Conductivity
The type of bonding can be determined by looking at if it's a good conductor of electricity. this chart shows conductivity based on phase
Solid | Aqueous | Liquid | Gas | |
|---|---|---|---|---|
Ionic | No | Yes | Yes | No |
Molecular covalent | No | no | no | No |
Network covalent | No | n/a | No | no |
Metallic | Yes | N/A | yes | No |
Covalent substances never conduct electricity, including pure water. Tap water is not pure water and is filled al dissolved ions that can conduct.
Ionic substances cannot conduct as solids because of the electrons stuck in the lattice structure. As liquids and when aqueous, the electrons can move around and conduct. The ability to conduct depends on concentration and now many ions they dissociate into.
1 M of NaCl conducts better than 0.1 M of NaCl because there's more ions present per unit of solution.
however, 1 M of CaCl2 would conduct better than NaCI because it dissociates into three ions rather than two ions.
Lewis Dot Structures
Drawing Lewis Dot Structures
Steps to drawing Lewis Dot Structure
Using the periodic table, count the valence electrons in the molecule
if it has a neg charge, add the amount of electrons equal to the charge. If it's positive, subtract
draw skeletal structure of molecule with the most electronegative in the middle and draw a pair electrons (single bond) between the elements.
Add electrons to each element until there's a complete outer shell.
add remaining electrons to central atom.
if central atom has less than eight electrons, remove pair from outer atom and make it a bond
if it's a complete octet, you're finished
Resonance Forms
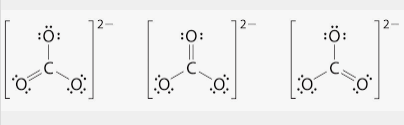
The Lewis dot structure of CO3 can be drawn with 2 single bonds and one double bond on any oxygen. Despite there being a double bond, each bond is a similar strength and length, in between a double and single bond.
To calculate the relative strength and length, you can use bond order calculations.
a single bond has a bond order of one and a double bond has a bond order of two. Add up the amount of single and double bonds and divide by now many resonance forms there are.
(1+2+1)/3= 1.33
Incomplete Octets
Some atoms do not need eight valence electrons. Hydrogen and helium only need two, but helium never bonds. Boron is stable with six electrons. All others need eight electrons in covalent bonds.
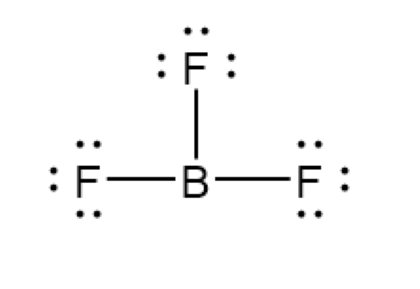
Expanded Octets
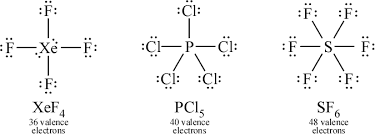
Molecules with d subshells can have more than eight valence, but never more than twelve. Silicon, phosphorus, sulfur, and chlorine can expand, but never carbon, oxygen, nitrogen. Noble gases can sometimes bond because of their empty d-orbital.
Formal Charge
Even though a molecule has several variations a Lewis dot structures, there is a more likely structure, called formal charge. To find formal charge, subtract the total number of valence electrons from the number of assigned electrons (bonded and lone pairs).
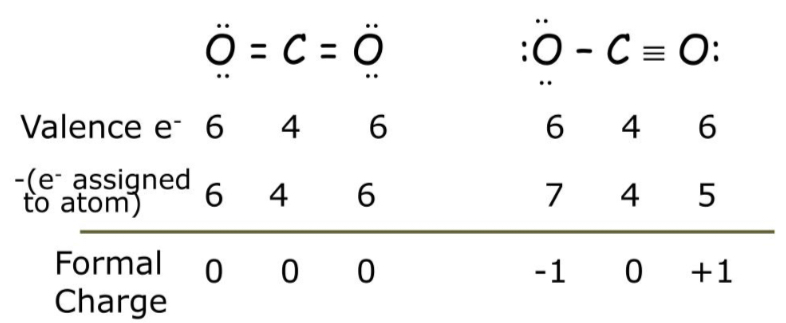
To determine which structure is more likely, choose the one with the formal charge of zero (the left structure on the diagram).
Molecular Geometry
Molecules will arrange in different shapes to keep electron pairs as far apart as possible to limit repulsion. To determine the shape, the valence shell electron pair repulsion model is used (VESPR).
When there's more than two electrons, the share depends on the number of bonds and lone pairs on the central atom.
Double and triple bonds are treated the same in terms of predicting overall geometry for a molecule
Lone electron pairs have a more repulsive strength than bonds.
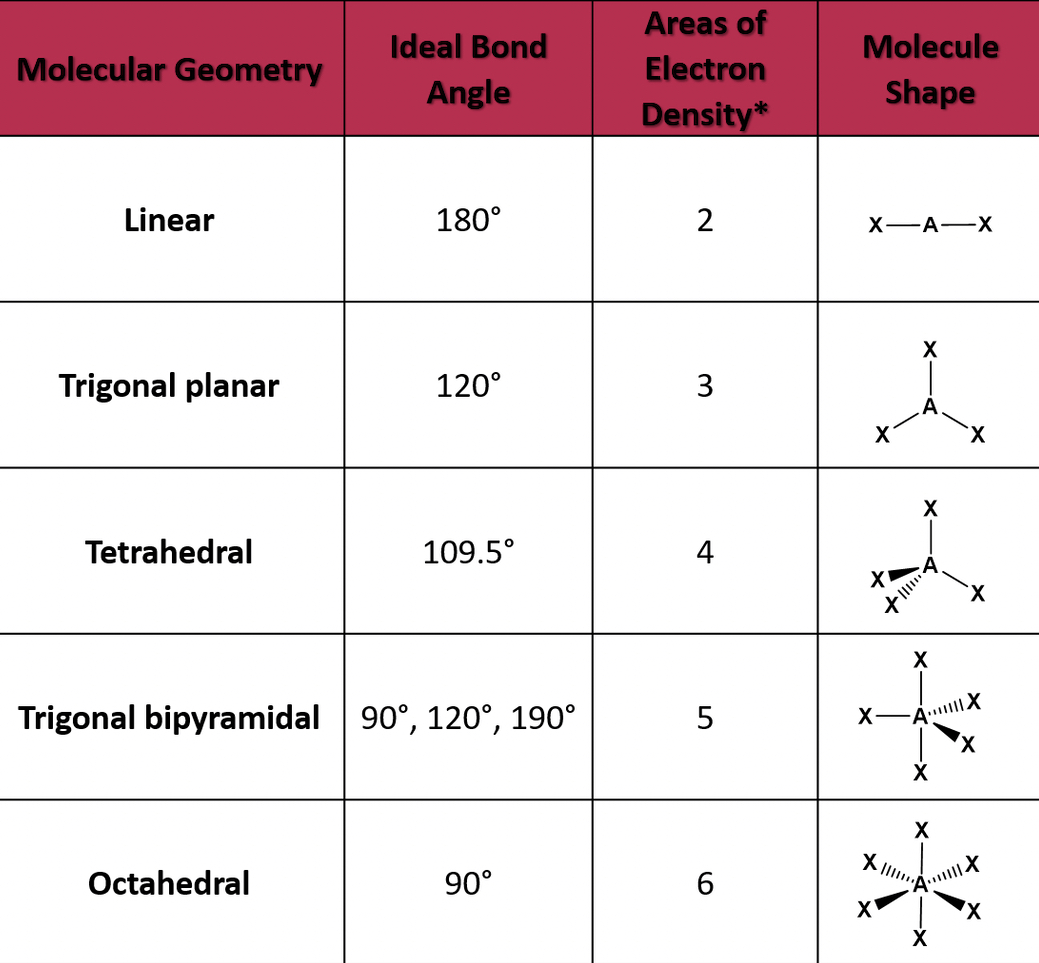
Types of geometries
If the central atom has two electron pairs, then it's an sp hybridization with a linear shape.
If the central atom has three electron pairs, it has the sp2 hybridization, and a basic shape of trigonal planar
The central atom has four electron pairs, it has an sp3 hybridization and a basic tetrahedral shape.
The central atom has five electron pairs, it has a trigonal bipyramidal shape.
The central atom has six electron pairs, its octahedral.
Unit Three: Intermolecular Forces and Properties
Polarity
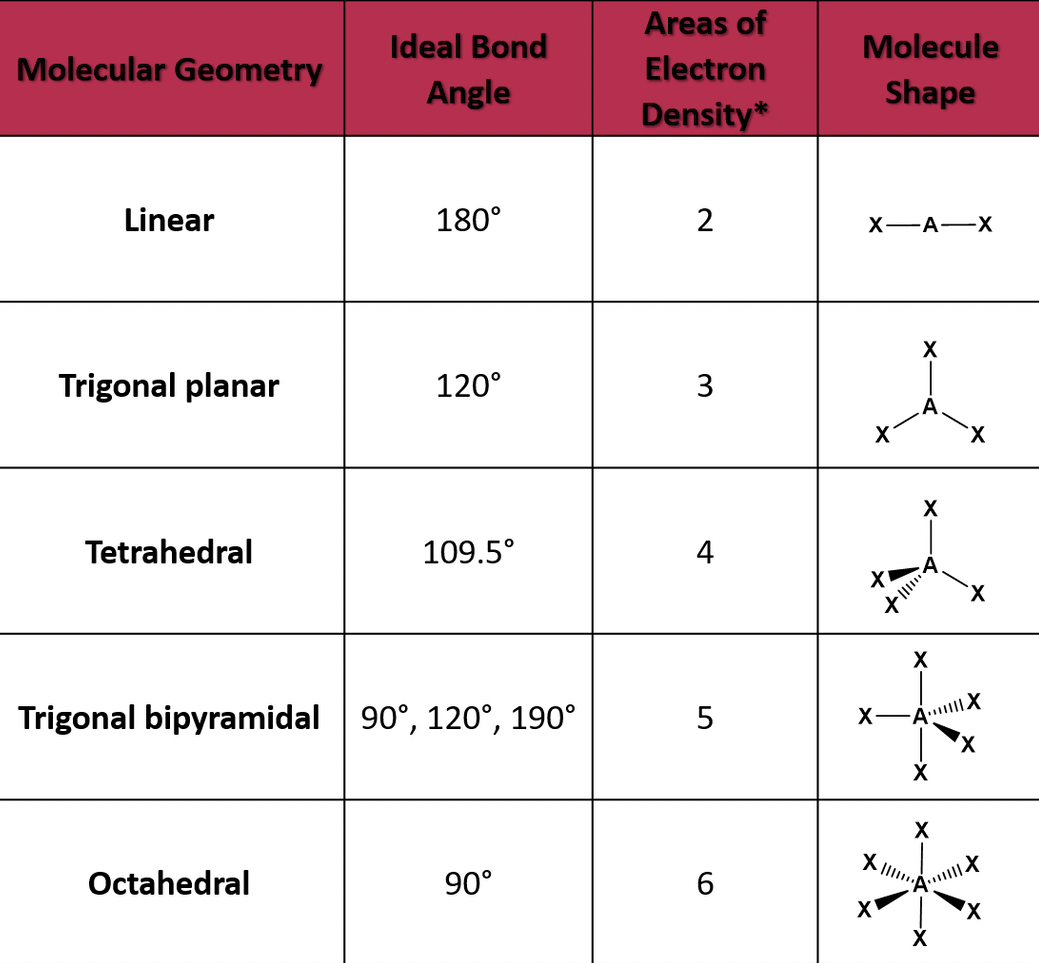
When a covalent bond is formed and electrons are shared, they are shared unequally. The more electronegative element in the bond has the stronger pull on electrons. The unequal share of electrons is called a polar covalent bond which also causes a slight partial charge on each atom.
When there's a slight charge, dipoles are created. Dipoles are opposite charges separated by some distance. Partial charges are indicated using the lowercase Greek letter delta.
When there's a covalent bond of equal or similar electronegativity, there is no dipole and it is a nonpolar covalent bond.
Molecular Polarity
Based on the type of molecular geometry and the dipoles of bonds, molecules can have polarity.
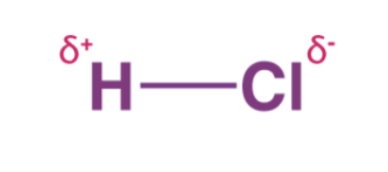
In carbon tetrafloride, the individual carbon-florine bonds are polar but because there are four C-F bonds and they are geometrically organized in a way that they cancel out, the molecule is nonpolar.
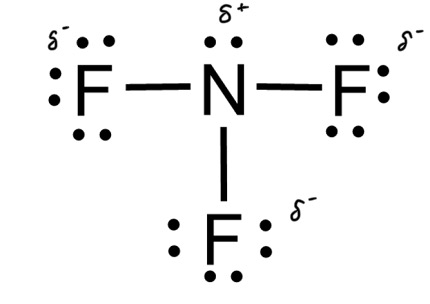
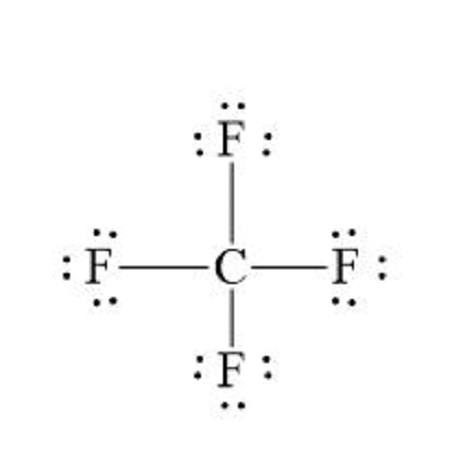
In NF3, only three of the four bonding locations has a fluorine, with the last being unfilled by a lone pair that belongs to nitrogen. fluorine is a more electronegative element than nitrogen, so it gets a negative charge.
Typically, if the central atom has a lone pair and different than the bonded atoms, it is asymmetrical and therefore polar. If the central atom has no lone pairs and all of the bonded atoms are the same, it is symmetrical and nonpolar.
lone pairs on the central atom making a molecule polar has a few exceptions: trigonal planar with three lone pairs or octahedral with two lone pairs.
Because in Lewis dot structures the least electronegative atom is in the center, polar molecules usually have a central positive charge. However, when hydrogen is a terminal atom, it's low electronegativity makes the central atom negative.
Intermolecular Forces
Intermolecular forces (IMFs) are the forces in between covalent bonds. in order for a phase change to occur, these forces must be broken.
when ionic substances change phases, the bonds between individual ions are actually broken.
when covalent substances change phase, the bonds between individual atoms remain but the bonds holding molecule to molecule together break apart.
Dipole-Dipole Forces
Dipole-dipole forces occur when the positive center of one polar molecule is attracted to the negative end of another polar molecule.
Molecules with greater polarity, and therefore greater dipole-dipole forces, have high melting and boiling points. However, dipole-dipole forces are weak and most substances held together by these forces are gases or liquids at room temperature.
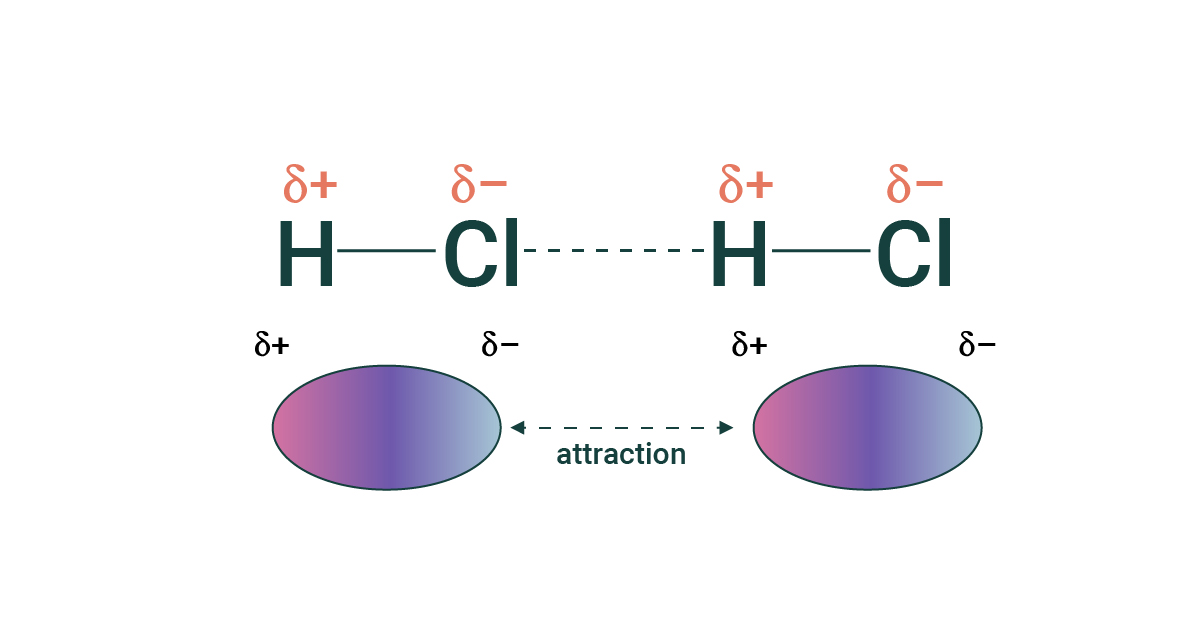
Hydrogen Bonds
Hydrogen bonds are a type of dipole-dipole force where the hydrogen (positive) end of a molecule is attracted to ONLY oxygen, nitrogen, or fluorine (negative end).
Hydrogen bonds are stronger than basic dipole-dipole forces because when hydrogen gives up it's electron, the nucleus in exposed. Water and ammonia have higher melting and boiling points than other molecules held together by non-hydrogen bonds.
London Dispersion Forces
London Dispersion forces (LDFs) occur between all molecules and are very weak attractions caused by random electron movements.
Because LDFs depend on random electron movement, a molecule with more electrons has stronger LDFs. Weak LDFs result in low boiling and melting points, ever lower and weaker than dipole-dipole forces. They are typically gases and liquids at room temperature.
even though LDFs are weak, it's hard to compare molecules with lots of electrons to polar substances with few electrons. LDFs may be equal to hydrogen bonds if there's enough electrons.
IMF Strength
To break the lattice structure of ionic solids and turn them into liquids, the bonds holding the lattice together must be broken.
Covalent substances are liquid at room temperature and will boil when their IMFs are broken. For similar size molecules, their IMF relative strength can be ranked.
hydrogen bonds are the strongest
nonhydrogen bond permanent dipoles
LDFs (more electrons = more/stronger (LDFs)
Covalent bonds are always weaker than ionic bonds
Metallic bonds tend to be very strong with high melting points, especially transition metals. Network covalent bonds are the strongest and are very hard to melt
Bonding and Phases
The stronger the IMF of a substance, the tighter the atoms are packed together. Solids have very tight-knit molecules and have the strongest IMFs. Gases have the weakest IMFs and have the most spread apart atoms.
substances with weak IMFs are gases at room temperature, such as nitrogen gas. Substances with hydrogen bonds are liquid at room temperature, such as water.
Ionic substances do not experience IMFs and have their phase determined by the ions in the bond. Ionic substances are usually solids at room temperature.
Vapor Pressure
IMF's can predict vapor pressure, the more movement there is in a liquid, the more likely it is to become a gas.The more kinetic energy, the higher likelihood of overcoming IMFs and changing phases.
Boiling is different than vaporization because boiling adds heat (energy) to cause the IMFs to break. Vaporization requires no excess heat to be added. However, the higher temperature of the liquid, the more likely the molecules are to break free of the IMF's
Stronger IMF's = lower vaporization.
Solution Separation
Using the different IMFs and Coulombic attractions of molecules, substances can be separated.
Solutes and Solvents
Like dissolves like.
polar substances will dissolve in polar solvents (salt in water). Nonpolar substances will dissolve in non polar solvents (oil). The dissociation, or breaking up, of ions can conduct electricity because they become electrolytes.
Paper Chromatography
Through chromatography, a mixture can be separated by passing it through a medium and each substance in the mixture reacts differently. A common form of chromatography is paper chromatography. in paper chromatography, paper is suspended with one end submerged in the solvent that slows climbs up the paper and pulls apart the solvent as it goes up.
Each substance has a different polarity and therefore a different attraction to the solvent. When black ink is separated via paper chromatography, the different colors in the ink are pulled apart. By determining which part moved the most with the solvent, you it can be determined which pert was the most polar or non polar (depending on whether or not the solvent is polar).
The distance of the ink travelled is measured by the retention factor (Rf) where Rf = (distance travelled by solute) /(distance travelled by solvent front).
Stronger attraction between solute and solvent front = stronger Rf value.
Column Chromatography
Two liquids pass through stationary substances and the distance travelled is determined by polarity.
Distillation
Two liquids with different boiling points are heated to the lowest temperature boiling point. The gaseous substance is collected and cooled to a liquid, which causes the two mixtures to be collected separately
Kinetic Molecular Theory
Ideal gases have certain rules:
The kinetic energy (KE) is directly proportional to the temperature. Higher temperature = higher KE.
Kinetic energy of a single gas molecule = 0.5 (mv)^2
m = mass of molecule in kg. v= speed of molecule in m/s. Ke measured in joules (j).
If different gases are present at the same temperature, they will have the same KE
The volume of a single ideal gas is negligible when compared to the volume the entire is contained in.
There are no forces of attraction between a gas molecules and an ideal gas.
Gas molecules are in constant motion, collides with the walls of the container and other gas molecules, and doesn't lose any energy.
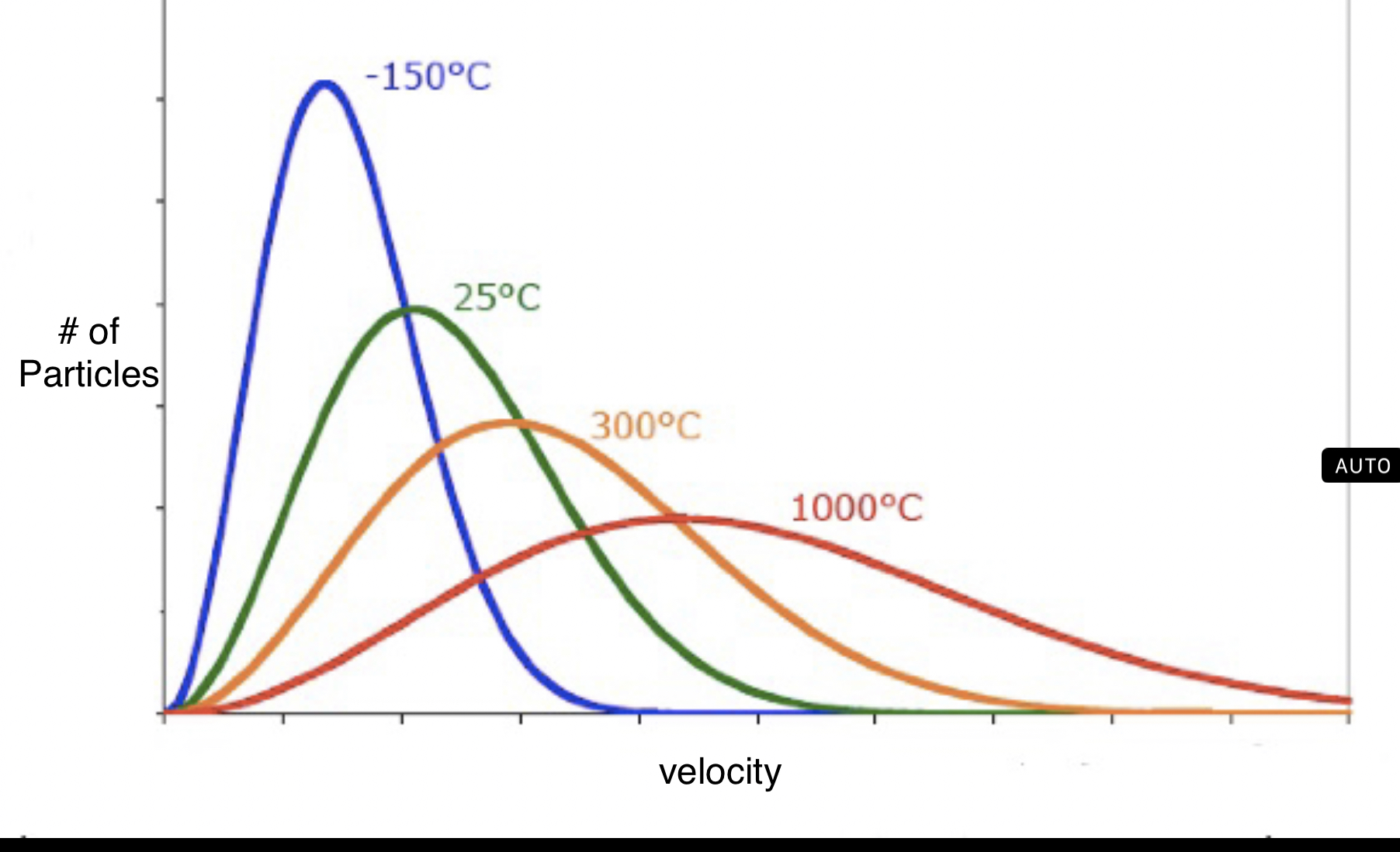
Maxwell-Boltzmann Diagrams
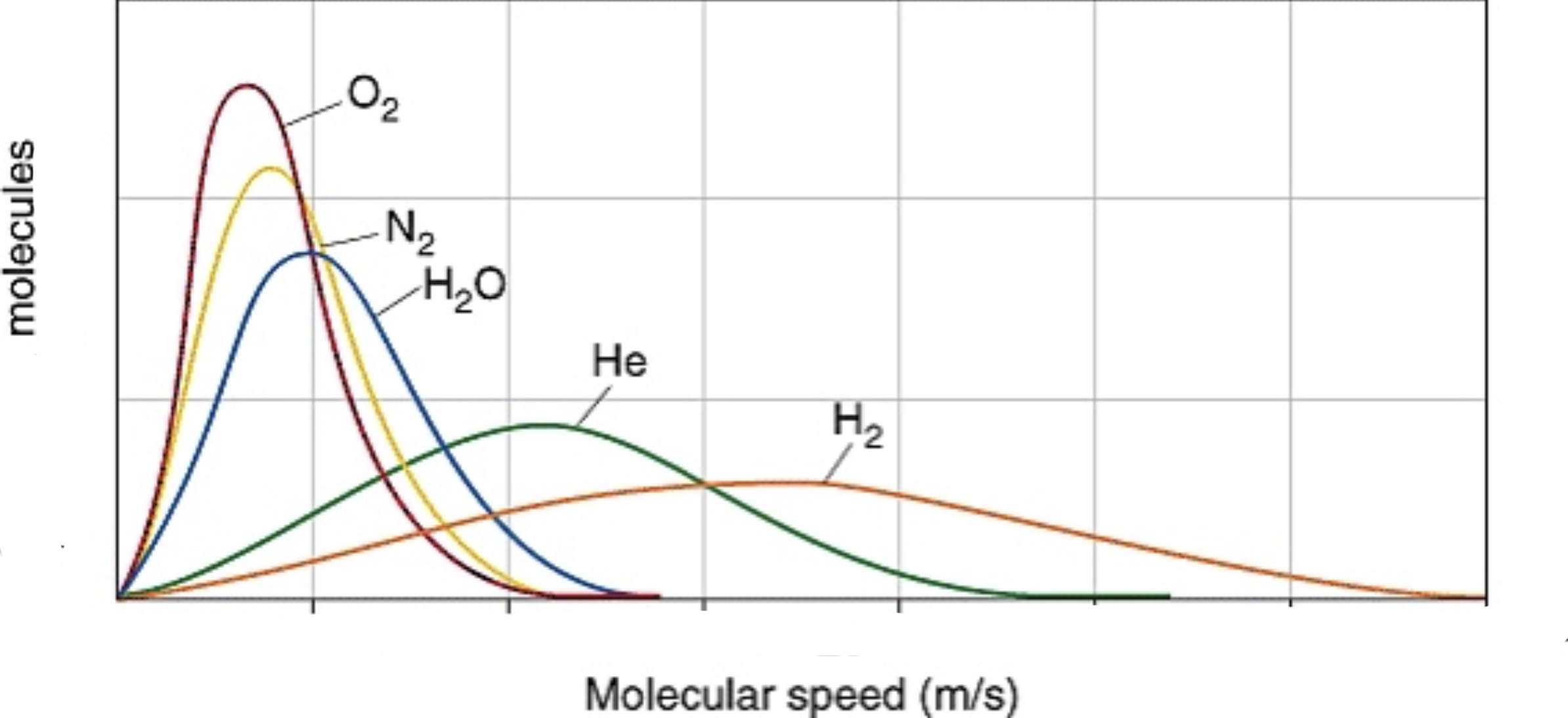
A maxwell-boltzmann diagram shows the range of velocities for molecules of a gas. Even though KE is the same for gases at the same temperature, this cannot be said for velocity because gas molecules have different masses.
The first type of diagram involves plotting the velocity of molecules for one gas at different temperatures. The higher the temperature, the higher variation of velocity in particles.
It can also show different gas velocities at the same temperature. Because oxygen gas weighs the most, it hasthe lowest velocity, but equal KE to hydrogen gas, which ways the least and has the highest velocity.
Effusion
Effusion is the rate a gas will escape from a container with microscopic holes from high pressure to low pressure. A balloon has tiny holes which is why gas escapes slowly over time.
Effusion rate depends on the speed of the molecule, temperature, and molar mass. The faster a gas is moving, the more collisions there are with the sides of the container, which makes them more likely hit a hole and escape. If the temperature is high, the rate of effusion is higher. A gas particle with a low molar mass will effuse before another gas with a higher molar mass.
The Ideal Gas Equation
The ideal gas equation is PV=nRT where P is pressure in atm, V is volume of the gas (L), n is the number of moles a gas, R is the ideal gas constant (.0821), and T is temperature in kelvin.
Kelvin can be determined by adding 273 to a temperature in Celsius.
The combined gas law is used when moles are held constant
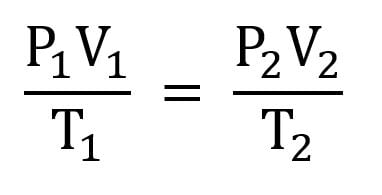
If the volume is constant: as pressure increases, temperature increases
If the temperature is constant: as pressure increases, volume decreases, according to Boyle's Law.
If the pressure is held constant: as temperature increases, volume increases, according to Charle‘s Law.
Dalton's Law
Dalton's law states that the sum all of the partial pressures of individual gases in a container is equal to the total pressure of gases in the container.
To find partial pressure of a gas in a container, divide the moles of that gas by the total moles of gas in the container and then multiply by the total pressure in the container. This is also called mole fraction.
Deviations From Ideal Behavior
The kinetic molecular theory becomes invalid when the temperature is too low and/or the pressure is too high because the gases become too tightly packed.
the volume of gases becomes significant because under high pressure the amount of volume of gas is taking up space that is significant when compared to the volume of the container.
gas molecules start sticking together which affects pressure since there's less molecules bouncing around.
Gases under normal conditions can deviate slightly if they have strong IMF's
Density
Density of a gas can be calculated using D=m/V where D is density, m is mass of a gas in grams, and V is volume in liters.
To find molar mass from density, use the equation MM=DRT/P
Electromagnetic Spectrum
An electron changing energy levels and the amount of electromagnetic radiation absorbed is determined by E=hv where E is energy change (in joules), h is Planck's constant (6.626 x 10^-34 j•s), and v is frequency (in s^-1).
wavelength can be determined from frequency
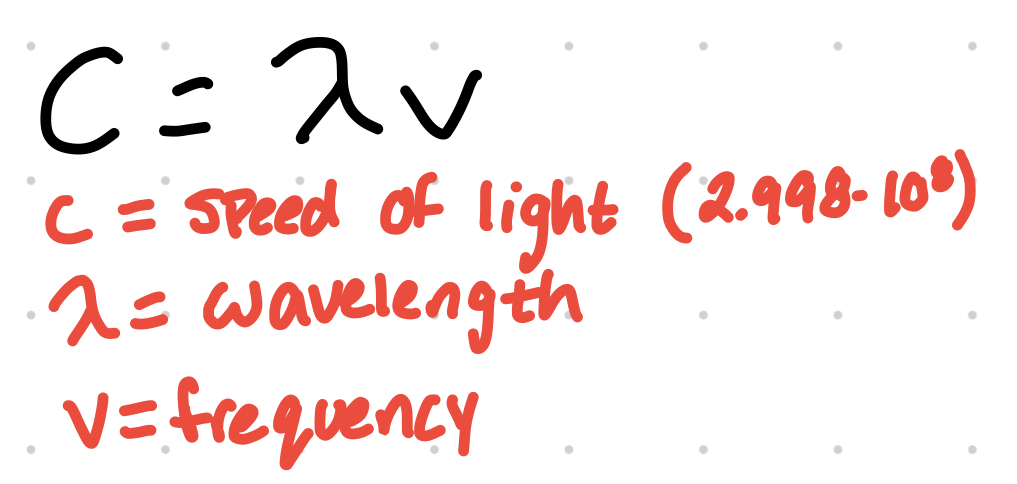
Beer’s Law
To measure the change of the concentration of a solution overtime, a spectrometer is used and can be calculated.
A=abc where A is absorbance, a is molar absorptivity of the solution, b is pathlength (how for light is traveling in the solution), and c is concentration.
Unit Four: Chemical Reactions
Types of Reactions
Synthesis reaction: when elements or simple compounds are combined to form a single, more complex compound. A+B → AB
Decomposition: opposite of synthesis-occurs usually in the presence of heat. AB → A + B
Acid-base: when an acid (H+) reacts with a base (OH-) to form water and a salt. HCl (aq) + NaOH (aq) → NaCI (aq) + H2O (l)
Oxidation-reduction (redox): results in change of oxidation state (only for some). Cu 2+ (aq) + 2 e- → Cu (s)
Hydrocarbon combustion: carbon + hydrogen (and sometimes oxygen) is ignited and produces CO2 and water, sometimes other compounds as well depending on the elements present. C4H10 + O2 → CO2 + H2O
Precipitation: two aqueous solutions mixing to create a solid precipitate. A (aq) + B (aq) → C (aq) + D (s)
Some solubility rules:
Compounds with alkali metals and ammonium are always soluble.
Compounds with nitrate are always soluble.
Chemical Equations
Balancing Chemical Equations and Calculations
Stoichiometry steps:
Covert given quantity to moles → use equation coefficients to determine limiting reactant → use balanced equation to determine how much product generated → convert moles to desired unit.
Percent error
% error = (|experimental value - expected value| / expected value) x 100%
Combustion Analysis
Because of the law of conservation of mass, when a hydrocarbon is combusted, all of the carbon in the hydrocarbon will create CO2 and all of the hydrogen will end up as H2O.
Gravimetric Analysis
Gravimetric analysis is used to determine an unknown element in a precipitate reaction.
A 4.33 g sample of an unknown alkali hydroxide compound is dissolved completely in water. A sufficient solution of copper (IL) nitrate is added to the hydroxide solution such that it will fully precipitate copper (Il) hydroxide via the following reaction: Cu 2+ (aq) + 2 OH- (aq) → Cu (OH)2 (s). After the precipitate is filtered and dried, its mass is found to be 3.81 g. Is the original alkali hydroxide sample most likely LiOH, NaOH. or KOH?
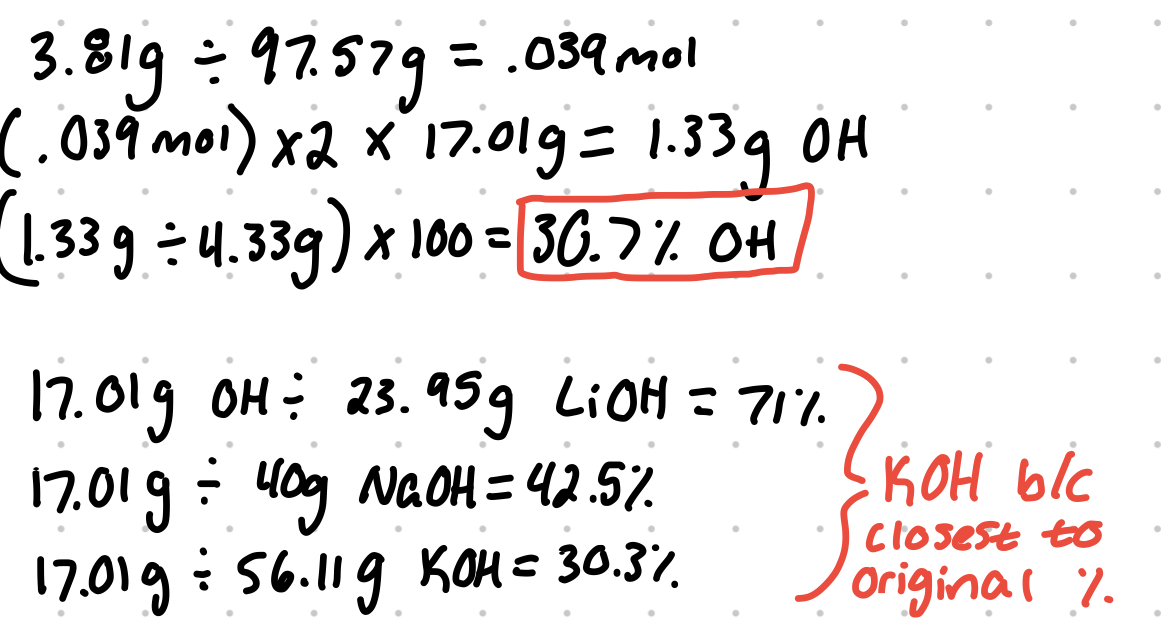
Oxidation States
In chemical reactions, electrons are transferred between reactants and can be determined with oxidation states, which have several rules.
Any neutral atom not bonded has an oxidation state of zero.
A singular atom has an oxidation state equal to it's charge.
In most compounds, oxygen is -2. One exception is H2O2, where oxygen is -1
When bonded to a nonmetal, hydrogen is +1. When bonded to a metal, hydrogen is -1.
When oxygen is not present, the most electronegative element has a state equal to it's most common charge.
The combined oxidation states must be equal to the charge of that compound
Oxidation-Reduction Reactions
In redox reactions, electrons are swapped between reactants and the oxidation states of the reactants are changed.
In Fe + 2 HCL → FeCl2 + H2, iron's state goes from 0 to +2 and H goes from +1 to 0.
Fe was oxidized
It can be written as a half reaction
Fe → Fe 2+ + 2e- oxidation
2 H+ + 2e- → H2 reduction
Redox Titrations
To determine the concentration of an unknown solution, a chemical added until usually a color change occurs.
If KMnO4 is added to a solution with MnO4 (which is a deep purple), the Mn is reduced and a color change occurs. If KMnO4 is added to a colorless solution that can be oxidized, it will turn pink.
Acids and Bases
An acid is a substance capable of donating a proton (H+) and a base is considered a substance capable of accepting a proton.
HC2H3O2 + H2O ⇌ C2H3O2- + H3O-
— HC2H3O2 and H3O are acids while H2O and C2H3O2 are bases
The H+ ion is the acid and the one without is the base. These are called conjugate pairs
HC2H3O2 and C2H3O2-
H2O and H3O-
However, water can sometimes act as an acid, this is called amphoteric
Unit Five: Kinetics
Rate Law Using Initial Concentrations
The rate law of a reaction is based on the initial concentrations of the reactants, the rate at disappearance of products and appearance of product, and requires experimental data. Arrhenius constant, k, is based on the activation energy for a reaction and the temperature.
How to do it
A+ 2B + C → D
experiment | initial [A] (M) | initial [B] (M) | initial [C] (M) | initial rate of formation of D (M/sec) |
|---|---|---|---|---|
1 | .1 | .1 | .1 | .01 |
2 | .1 | .1 | .2 | .01 |
3 | .1 | .2 | .1 | .02 |
4 | .2 | .2 | .1 | .08 |
Rate = k[A]^x[B]^y[C]^x
The value of the exponent is based on how the reactants affect the rate. Larger exponent means the reactant concentration changes more. To find the exponent value, find what happens when a concentration is doubled.
For [A]
Use experiment 3 and 4 values for [A] because no other reactant value changes. The rate changes from .02 to .08 by quadrupling. The value of the exponent x is found by doubling the concentration and setting it equal to the value of the rate changes (4).
(2)^x = 4, so x=2.
Now the rate is Rate = k[A]^2[B]^y[C]^x or second order with respect to A.
For [B]
Use experiment 1 and 3. it doubles the rate from .01 to .02.
(2)^y=2, so y=1
The rate is now Rate = k[A]^2[B]^1[C]^x or first order with respect to B
For [C]
Using experiment 1 and 2, the rate stays the same so x=0
Rate = k[A]^2[ B]^1[C]^0 or Rate = k[A]^2[ B]^1 so the reaction is overall third order.
Any experiment can be used to find k. Using experiment 3:
k = (rate/[A]^2[B]) = (.02/(.1)^2(.2)) = 10 M^-2sec^-1
Rate Law Using Concentration and Time
Rate law can be expressed using concentration changes over time. There are zero, first, and second order rate laws that each have the roan equation and graph.
Zero-Order Rate Laws
In zero order, the rate is always the same at a given temperature and doesn't change based on reactant concentration.
Rate = k
The graph is a straight line equal to -k and concentration vs. time.
First- Order Rate Laws
First order rate law is based on the concentration of one reactant to the first power.
Rate = k[A]
The graph is the natural log of [A] vs. Time. This creates a straight line with a slope of -k and y-interest of ln[A] at time 0.
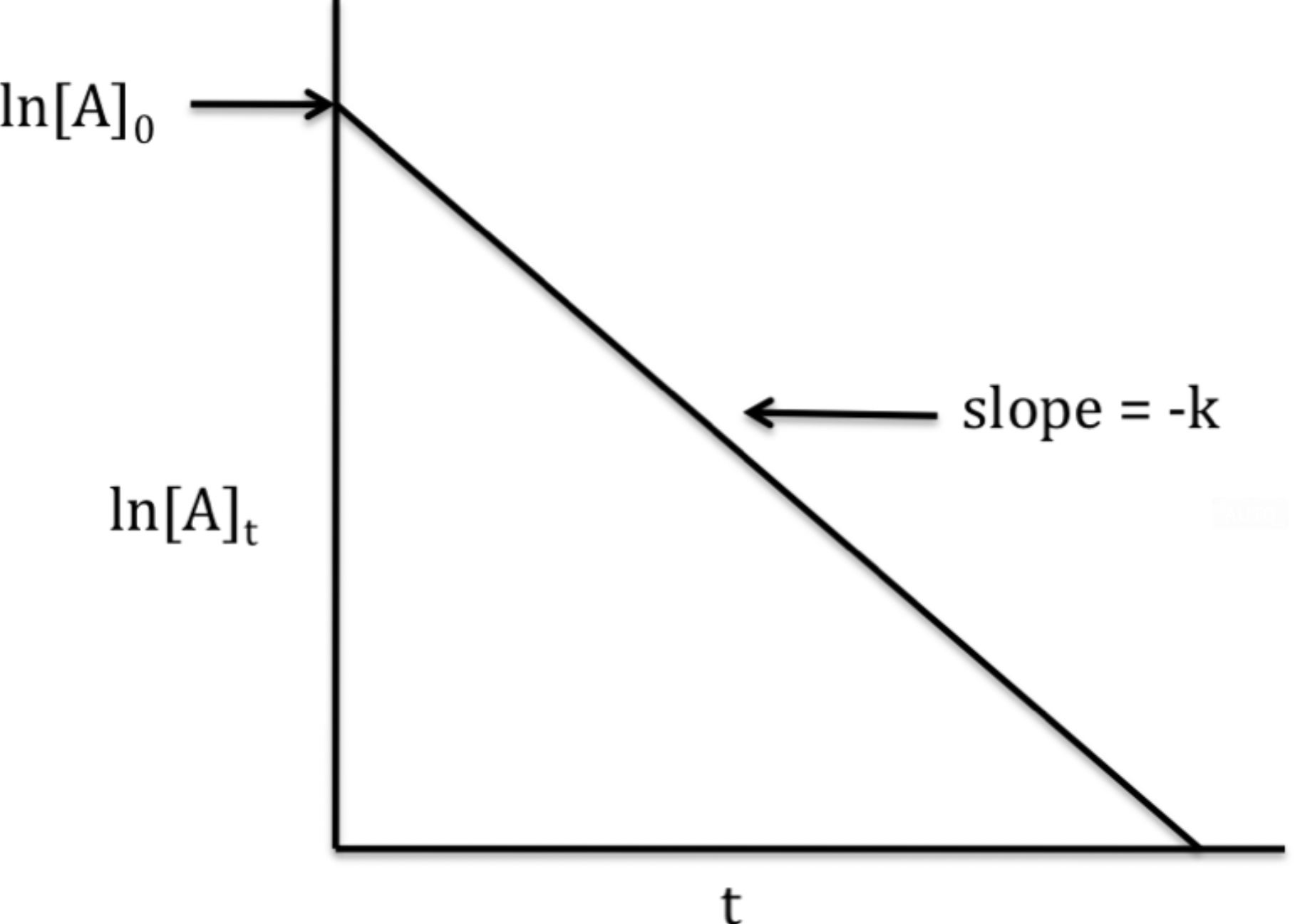
The rate law, using the slope-intercept formula, is

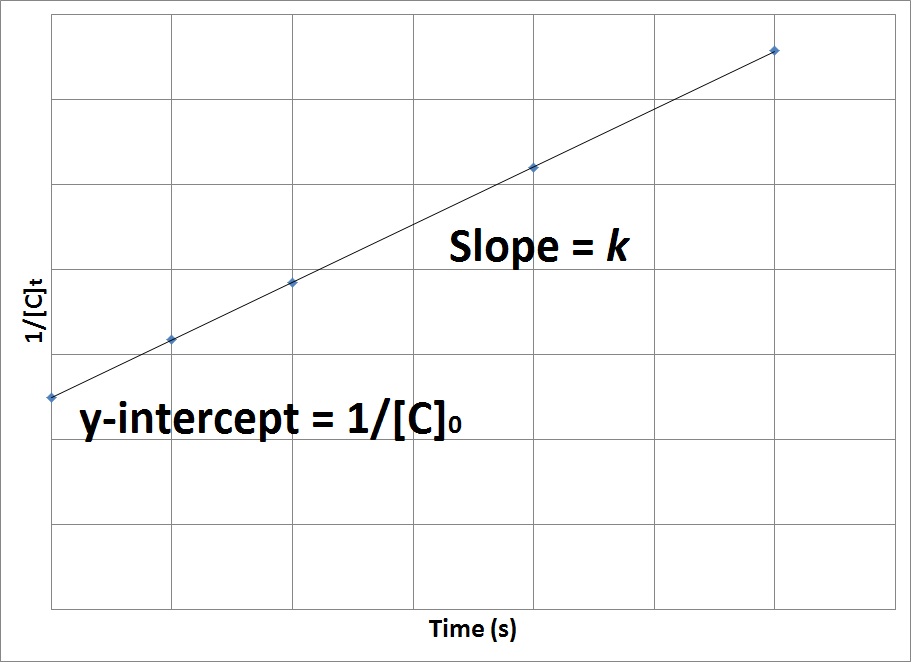
Second-Order Rate Law
Second-order rate depends On a reactant raised to the second power.
Rate = k[A]^2
The rate law uses the inverse of concentrations.
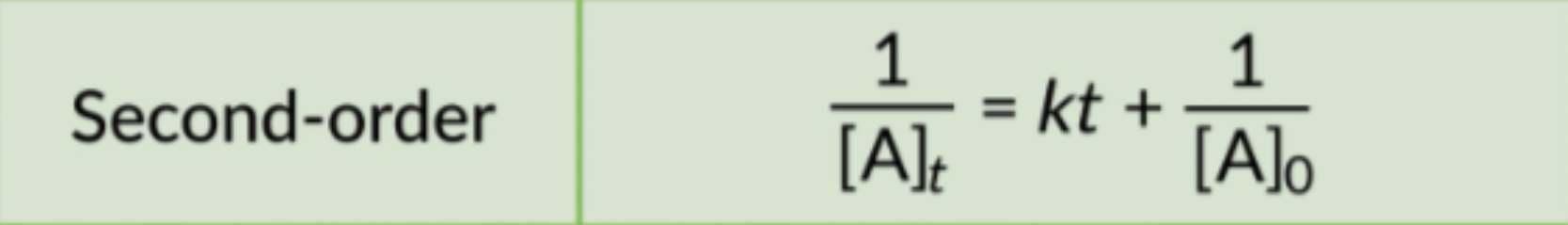
The inverse concentrations vs. time create a straight line, where the slope is k and y-intercept is (1/[A]) at time zero.
Half-Life
Half-life of a substance is how long it takes for half of the substance to react.
Time | Sample |
|---|---|
0 half lives | 100% |
1 half life | 50% |
2 half lives | 25% |
3 half lives | 12.5% |
In first-order, half-life is constant. If after 30 sec 50% of a substance has decayed, in 30 more sec, 25% remains. 30 more sec, 12.5% remains, etc.
Half-life can be determined graphically
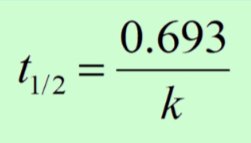
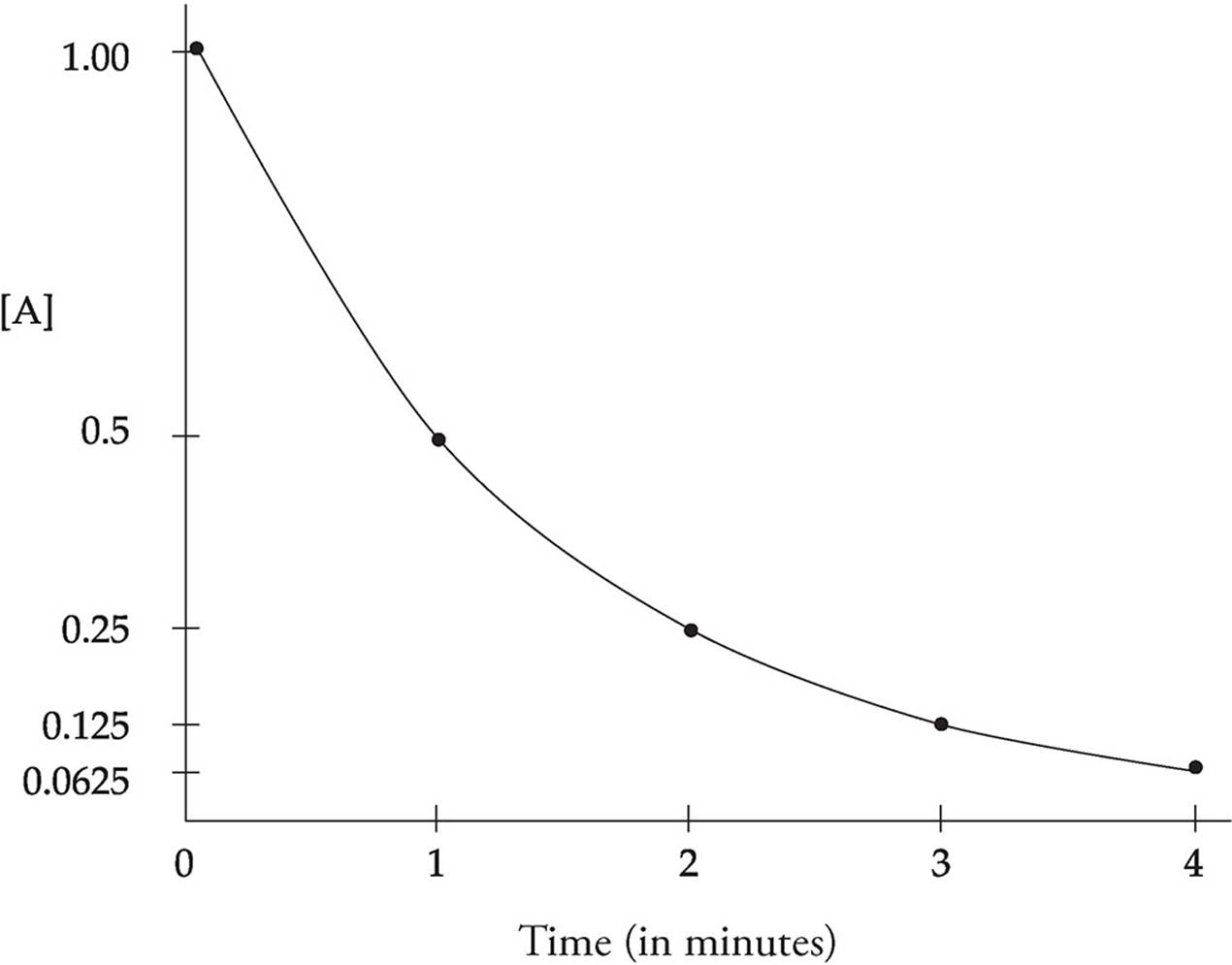
The half-life equation can only be used for first-order reactions because zero and second order do not decompose at the same rate and changes overtime
Collision Theory
Collision theory states reactions only occur when chemicals collide with each other with sufficient energy (activation energy).
They can react more if there's a higher concentration of aqueous or gaseous substances, or a high surface area of a solid substance.
Stirring can sometimes speed up a reaction. If the mixture is heterogeneous, not all parts of a mixture is identical, then stirring will mix it more and speed it up. If the mixture is homogeneous, where the mixture is completely identical, then stirring will not help because it is already mixed and even.
Reaction rate increases with temperature because molecules are moving faster and have a higher likelihood to collide with other molecules at a sufficient speed.
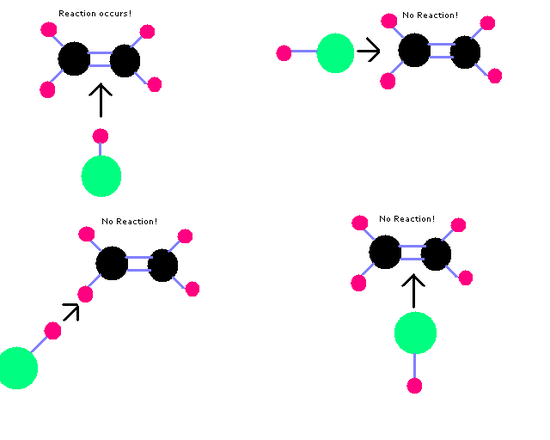
Molecules will only react if they collide with the correct orientation.
Reaction Energy Profile
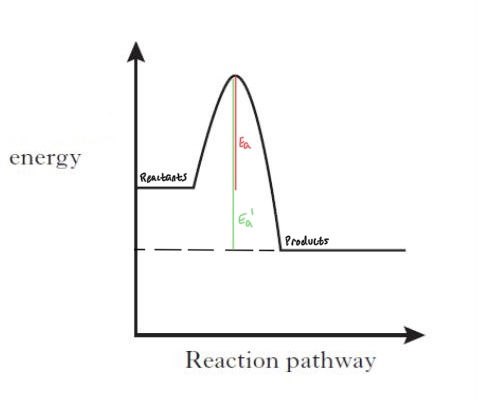
The diagram shows a chemical reactions energy change. The red line is the activation energy, or how much energy is required for the reaction to occur. The green line is how much energy required for the reverse reaction.
Reaction Mechanisms
Some reactions occur in multiple steps rather than in one step and the overall balanced reaction is the sum. The in between steps are called elementary steps.
Example: The rate for elementary steps can be determined by setting the concentration of a substance to the power of it’s coefficient
I. A + A ⇌ X (fast) Rate=k[A]^2
II. X + B → C + Y (slow) Rate=k[X][B]
III. Y + B → D (fast) Rate=k[Y][B]
X and Y are intermediates, they are created in one step and consumed in another, so they cancel out.
If an elementary step has only one reactant, it is unimolecular. If it has two reactants (even if they are the same reactant, like in step l), it is considered bimolecular.
The slowest step is considered the rate-determining step because the speed of the reaction cannot exceed the slowest step. This step determines the rate law for the entire reaction.
Step II has an intermediate, which is not written in the reaction, and it is produced in step I, so X can be replaced with [A]^2. The rate law for step two and the overall reaction becomes Rate=k[A]^2[B].
a multi - step reaction can be written as a reaction energy profile diagram
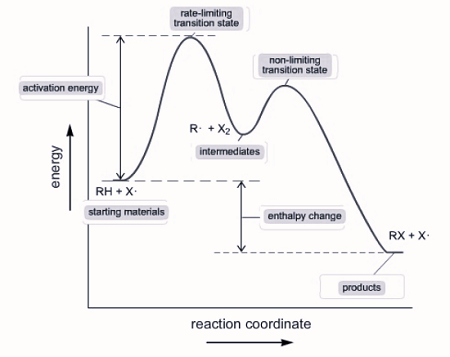
Catalysts
Catalysts are added to a reaction to speed it up without being consumed. Catalysts are present in the beginning and end of elementary steps and cancel out.
For the steps in the reaction A + B → C
I. A+ X→ Y
II. B + Y → C + X
In this reaction, x is the catalyst and Y is the intermediate
Unit Six: Thermodynamics
Heat and Temperature
Temperature is the average amount of kinetic energy based on molecular motion of a substance
Heat is the flow of energy between two substances at different temperatures
The first law of thermodynamics states energy cannot be created or destroyed and can only transfer or change forms. Energy transfers through molecular collisions.
Exothermic is when energy is transferred from the system to the surroundings, such as when a chemical reaction occurs in a beaker and the beaker feels hot. Endothermic reactions occur when a system absorbs the energy from the surroundings, such as when a beaker feels cold.
Enthalpy
Enthalpy Change, ΔH
Enthalpy change is the measure of energy that is released or absorbed when bonds are broken/formed
When bonds are formed, energy is released. When bonds are broken, energy is absorbed.
If more energy is released than absorbed in a reaction, then it is exothermic and has negative enthalpy change. If more energy is absorbed than released in a reaction, then it is endothermic and has a positive enthalpy change.
Energy Diagrams
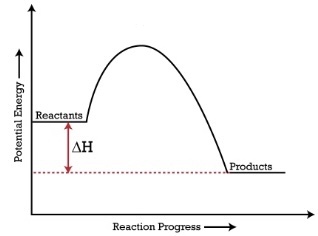
Exothermic and Endothermic Reactions
When products have a lower energy than the reactants, the ΔH is negative and it is an exothermic reaction
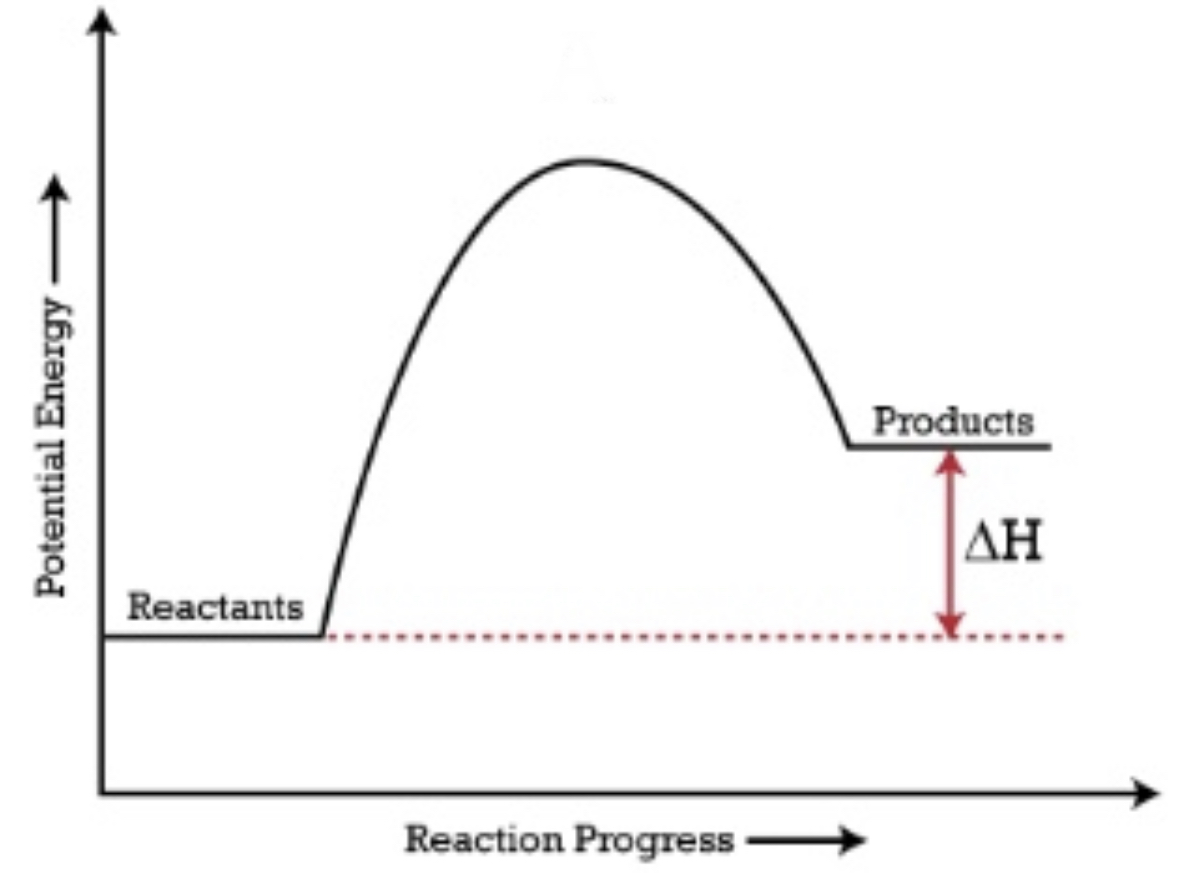
When reactants have a lower energy than the products, the ΔH is positive and it is an endothermic reaction
Catalyst and Energy Diagrams
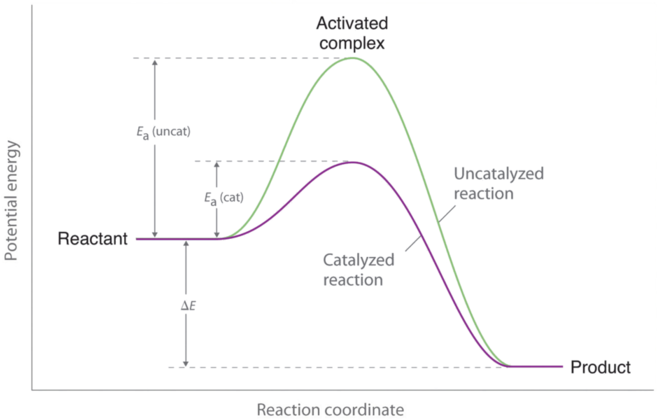
Catalysts speed up a reaction by lowering the required activation energy and only changes the activation energy, not the amount of energy of products or reactants in the end or beginning (ΔH).
This change can only occur when a reaction is not in equilibrium conditions
Enthalpy of Formation, ΔHf°
Enthalpy of formation, or heat of formation, is the energy change when one mole of a compound is formed from pure elements under standard conditions (298°K, 1 atm)
Example: C(s) + 1/2 O2 (g) + 2 H2 (g) → CH3OH
There is exactly one mole of a product, methanol, which has an odd number of elements, and diatomic in nature.
ΔHf° of a pure element is defined as zero and stands true for elements that are diatomic in pure states, such as hydrogen or oxygen.
If ΔHf° of a compound is negative, energy is released when the compound is formed from pure elements, is exothermic, and is more stable than the reactants.
If ΔHf° is negative, the opposite is true, and it is endothermic.
ΔH can be found if ΔHf° of product and reactants are known
ΣΔHf° Products - ΣΔHf° Reactants = ΔH
The units for reactions enthalpy is kj/mol.
Bond Energy
Bond energy is the energy required to break a bond and is always a positive number. When a bond is formed, the energy of formation is equal to the bond energy released.
ΔH = Σ Bond energy of bond broken - Σ bond energy of bonds formed
Bonds broken are the reactant bonds and the bonds formed are the product bonds
The number of bonds broken is determined by how many moles of that substance there is and how many individual bonds there are.
For 2 H2O molecules, there are two H-O bonds and two of the H2O molecules. So, there are four H-O bonds and to find the bond energy, multiply four by the bond energy of H-O (463 kJ/mol).
Hess’s Law
Hess’s Law states that if a reaction can be broken into a series of steps, the sum of the ΔH of those steps are equal to the ΔH of the reaction.
When manipulating equations for enthalpy calculations…
If a reaction is flipped, flip the enthalpy value (pos to neg, or neg to pos)
If you multiply or divide the coefficients of a reaction, multiply or divide the enthalpy value by that same interval
Adding up the enthalpy values of steps will be the new enthalpy value for the new reaction created from those steps
Enthalpy of Solution
When an ionic substance dissolves in water, energy is absorbed because bonds are breaking, but energy is also emitted because new dipoles are forming.
Step one of dissolving: Bonds are broken in the solute. This step has a positive ΔH because bonds are being broken
Step two: solvent molecules are separated. The water molecules are spread apart to make room for the solvent ions. This weakens intermolecular bonds, resulting in a positive ΔH
Step three: New attractions are created. The free-floating ions are attracted to the dipoles in the water molecules, resulting in a negative ΔH despite no bonds being formed.
Step two and three values are combined to form the hydration energy which is always a negative number. Hydration energy is a Coulombic energy and it’s magnitude increases as the ions increase their charge or decrease the size.
If adding all three steps together, the enthalpy of solution can be determined.
Thermodynamics of Phase Change
Naming the Phase Changes
Solid to liquid: melting
Liquid to solid: freezing
Liquid to gas: vaporization
Gas to liquid: condensation
Solid to gas: sublimation
Gas to solid: deposition.
Vapor pressure is the amount of pressure given off by molecules as they go from solid/liquid to gas.
Enthalpy of Fusion
Enthalpy of fusion is the amount of energy required to cause a solid to melt. Heat of fusion is how much heat given off when a substance freezes. The intermolecular forces in a solid substance are more stable when they are solid so they have a lower energy than liquid forces, so energy is released in the freezing process.
Enthalpy of Vaporization
Enthalpy of vaporization is how much energy is required to turn a liquid to a gas. The heat of vaporization is the energy given off when a gas condenses. Intermolecular forces are more stable in a liquid state, so energy is released as the forces stabilize and get stronger.
When heat is added to a substance, it will either phase change or increase in temperature, not both. Only one can happen at a time. Temperature remains constant during a phase change.
Calorimetry
Calorimetry is the measurement of heat changes during a chemical reaction
The specific heat of substance is how much heat is required to raise one gram of a substance by one degree celsius (or one degree kelvin). The higher or larger a specific heat is, the more heat a substance can absorb without changing temperature. A low specific heat means less heat is required to change the temperature of a substance.
Specific heat equation is q = mcΔT
q is heat added (J or cal)
m is the mass of the substance (g or kg)
c is specific heat
ΔT is temperature change (K or C
Unit Seven: Equilibrium
The Equilibrium Constant, Keq
In equilibrium reactions, the reaction is reversible, where the reactants from products and then go back to reactants constantly.
In the Haber process, the industrial production of ammonia, N2+3H2 ⇌ 2NH3, the reaction is reversible.
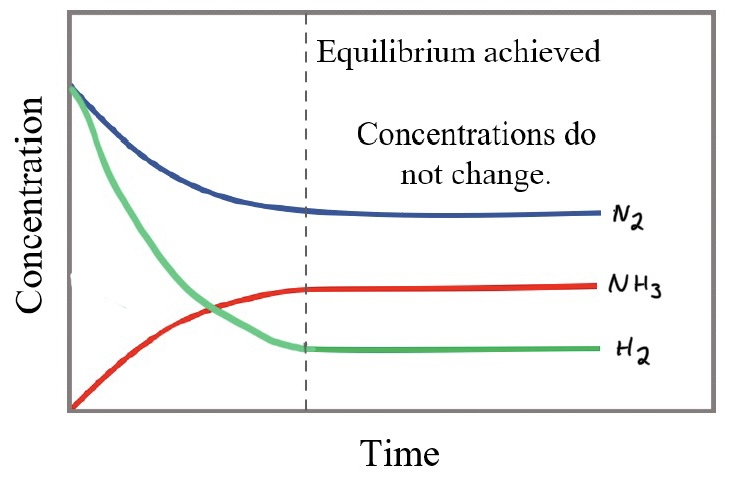
At the equilibrium point, the amount of reactants and products has stopped changing, but the reaction is still occurring, the forward and reverse reactions are equal.
The coefficients determine at what rate a substance is created or consumed. The H2 is consumed three times faster than the N2, so it is a steeper slope when they are initially the same concentration.
The Law of Mass Action is the equilibrium expression that determines the relationship between reactant and product concentrations.
Equilibrium expression for the reaction aA+bB ⇌ cC+dD
Keq = [C]^c[D]^d / [A]^a[B]^b
[A], [B], [C], [D] are the molar concentrations or partial pressures at equilibrium
Divide the products by the reactants
Only gasses and aqueous solutions are in the equilibrium expression
No units for Keq
A Keq > 1 means the products are favored over reactants. A Keq < 1 means reactants are favored over products.
Different types of K values, or equilibrium constant
Kc is constant for molar concentrations
Kp is partial pressure constant
Ksp is solubility product constant, which has no denominator because reactants are solids
Ka is the acid dissociation constant
Kb is base dissociation constant
Kw is the ionization of water (Kw is 1.0 x 10^-14)
Manipulating Keq
Hess’s law can be applied to equilibrium equations
If a reaction is flipped, take the inverse of the equilibrium constant
If you multiply the coefficients, take the constant to the power (multiply by two, square the constant)
If you add reactions together, multiply the constants together to get the new constant
Le Chatelier’s Principle
Whenever a stress is placed on a system, the reaction will shift to go back to equilibrium. If a reaction shifts left, it will create more reactants. If it shifts right, it will make more products.
Concentration
If concentration is altered, the reaction will shift to use up more or less of the substance with an altered concentration.
For the Haber process, if N2 or H2 is added, it will shift right to create more NH3 and use up more of the reactants. If more NH3 is added, the reaction will shift left to create more N2 and H2.
If N2 or H2 is removed, it will shift left to make up for the loss of the reactant and create more reactants.
Pressure
When external pressure is increased, the partial pressure of gases inside a container will increase, and the reaction will shift to the side with less gas molecules. Decreasing the partial pressure will cause the reaction to shift to the side with more gas particles
If the pressure of a container is increased, the reaction will shift to the right where there are 2 moles of gaseous products rather then the left, where there are four moles of gaseous reactants.
If the pressure is decreased, the reaction will shift left where there’s more moles of gas.
If a noble gas is added at a constant pressure, the equilibrium reaction will shift to the side with more gaseous molecules. If a noble gas is added to a reaction at a constant volume, no shift occurs.
Temperature
The Haber process has a ΔH of -92.6 kj/mol, so it is exothermic, so heat is generated, this creates the equation:
N2+3H2 ⇌ 2NH3 + energy
If the temperature goes up, the reaction will shift to the left, making more reactants. If the temperature goes down, the reaction would shift to the right and produce more products
Dilution
If an aqueous equilibrium is diluted, such as the equation Fe 3+ (aq) + SCN- (aq) ⇌ FeSCN 2+ (aq), diluting an equation with extra water will cause a shift.
If this equation was diluted, it would shift the side with more aqueous molecules, the left. If water evaporated, it would shift to the side with less aqueous molecules, or the right.
Changes in the Equilibrium Constant
When concentration or pressure changes, the equilibrium constant stays the same and the product to reactant ratio will revert to the original value.
When temperature is changed, the equilibrium constant is changed and will be a new value because it affects the kinetics and the equilibrium constant.
The Reaction Quotient, Q
The equilibrium constant can only be used when equilibrium is achieved, but the reaction quotient, Q, can be used at any point in time during a reaction to determine how close or far the reaction is to equilibrium and how it will shift.
The equation is the same as the Keq equation.
If Q is less than K, the reaction shifts right.
If Q is greater than K, it will shift left
If Q and K are equal, the reaction is at equilibrium.
Solubility
A salt is considered soluble if one gram of the salt is dissolvable in 100 mililiters of water. Soluble salts are assumed to have completely dissolved in an aqueous solution. Most solids increase in solubility as the temperature increases.
Solubility Product (Ksp)
Ksp is the measurement of how much a salt dissolves in a solution. The larger the Ksp value is, the more soluble the salt is.
For the reaction AaBa (s) ⇌ aA (aq) + bB (aq)
The solubility expression is Ksp= [A]^a[B]^b
The solubility of a salt is equal to the ion concentration it dissolves into.
If the molar solubility of the salt AaBa is 1.3x10^-4 M, then the A ion concentration is the same as the solubility time the value a. (Keep in mind the salt to ion ration)
Typically, as temperature increases, the increase in molecular motion allows the salt to dissolve more, but sometimes it will decrease as temperature increases. This is hard to predict.
The Common Ion Effect
If AgCl is put into a solution to dissolve, it will only do so in small amounts. If NaCl is added to the solution, it will dissolve completely and not affect the Ag ions. The Na ions can be ignored but all of the Cl ions from the NaCl and AgCl must be considered because of the common ion effect.
According to the common ion effect, the new Cl ions will affect the AgCl equilibrium even though the Cl ions came from NaCl.
Unit Eight: Acids and Bases
pH
Concentration equations are usually in pH
pH = -log[H+]
pOH = -log[OH-]
pKa = -log[Ka]
In a solution
If [H+] = [OH-] the solution is neutral with a pH is 7
If [H+] > [OH-] the solution is acidic wih a pH of <7
If [H+] < [OH-] the solution is basic with a pH >7
Increasing pH means decreasing [H+] and making a solution less acidic.
Decreasing pH means increasing [H+] and making a solution more acidic.
Some hydroxide salts will not dissolve in high pH because of the common ion effect.
In the equation Mg(OH)2 (s) ⇌ Mg2+ (aq) +2OH-(aq)
If the salt is added to a solution with significant hydroxide ions in it, it will shift to the left and decrease the amount of salt dissolving. If added to a solution with an abundance of hydrogen ions, it will dissolve more because the hydrogen and hydroxide will react, creating less hydroxides in the solution, and the salt to dissolve more.
Acid Strengths
Strong Acids
Strong acids will dissolve completely in water and never reach equilibrium. There is no equilibrium constant for strong acids or bases because of no equilibrium
Important strong acids
HCl, HBr, HI, HNO3, HClO4, H2SO4
Important strong bases
LiOH, NaOH, KOH, Ba(OH)2, Sr(OH)2
Because completion occurs, the conjugate base of the acid must be extremely weak.
Finding the pH of strong acids is easier than finding the pH of weak acids because strong acids will dissociate completely while weak acids will only partly dissociate so the concentration of hydrogen ions is different in the beginning versus the end
Weak Acids
A weak acid in water will have a small fraction dissociate into the conjugate base and hydrogen ions while the rest remains undissociated aqueous particles.
Weak acid and base dissociation constants are Ka and Kb, specific to only acids and bases
Ka = [H+][A-]/[HA]
[H+] is hydrogen ion concentration in molarity
[A-] is conjugate base ion concentration in molarity
[HA] is concentration of undissociated acid molarity
Kb = [HB+][OH-]/[B]
[HB+] is conjugate acid ion concentration
[OH-] is hydroxide ion concentration
[B] is unprotonated base molecules
Greater Ka value means more acid dissociation (stronger acid)
Greater Kb value means more protonatization (stronger base)
Bases do not dissociate but accepts protons
Percent Dissociation
More hydrogen ions an acid can donate means stronger acid and the ease of which an acid can dissociated is determined by molecular structure
For binary halogen acids, HI, HBr, and HCl are all strong acids but HF is not because of its high electronegativity
For oxacids, the reverse is true
For HOF, the hydrogen and fluorine are on opposite ends, so the hydrogen is less electron dense because the oxygen acts as a buffer and can dissociate
For HOBr, the hydrogen is slightly more electron dense than the hydrogen in the HOF because Bromine isn’t as electronegative as the fluorine
The more oxygens you add to an oxacid, the stronger of an acid it becomes.
If there is an abundance of water with an acid, or a smaller concentration of acids, the acid can find a water to donate the hydrogen to and will react/dissociate more
If there is a greater concentration of an acid, or less water molecules, it will not be able to dissociate as much because there is less water to be able to accept a hydrogen ion
Acid/Base Structure
Only some hydrogens in an acid are able to dissociate
This is why acetic acid is written as HC2H3O2 rather than C2H4O2
The hydrogen will dissociate from the least electronegative end.
However hydrogens attached to a carbon nearly never dissociate because they share the electron fairly equally because of the similar electronegativity.
Generally, hydrogens that are written in the beginning of an acid are able to dissociate while ones in the middle cannot
pH and Solubility
Mg(OH)2 (s) ⇌ Mg2+ (aq) +2OH-(aq)
If the salt is placed in a high pH, it would be less soluble than if it was placed in a lower pH.
Higher pH means more hydroxide ions and cause the salt to be less soluble/react less
Lower pH means more hydrogen ions and cause the salt to dissociate more/be more soluble
This is similar to the common ion effect
Polyprotic Acids
Polyproticacids can give up more than one hydrogen in a solution
H2SO4 and H3PO4 are polyprotic
The first proton is easier to give up than the second one
The first Ka for H3PO4 is 7.1x10^-3
The second Ka for H2PO4 is 6.3x10^-8
The third Ka for HPO4 is 4.5x10^-13
H3PO4 is stronger of an acid thand H2PO4 because the protons are more attracted to the H2PO4 and not as easily given up
Equilibirium Constant of Water (Kw)
Water comes to equilibrium through the reaction
H2O (l) ⇌ H+(aq) + OH-(aq)
Kw = 1x10^-14 at 25 degrees celsius
Kw = 1x10^-14 = [H+][OH-]
pH + pOH =14
The H and OH in any acid or base solution must be consistent with the ionization of water
Ka and Kb values are also consistent with the water equilibrium reaction
Kw = 1x10^-14 = Ka x Kb
pKa + pKb =14
pHs can exceed 14 and be lower than 0, but are uncommon
The Kw constant is only at 1x10^-14 at 25 degrees celsius because bonds will form and break as the temperature changes so the Kw value changes
Neutralization Reaction
Neutralization reactions occur when an acid donates its proton to a base
Strong acid + strong base
Both substances dissociate completely and the only important ions in this reaction are the hydrogen and hydroxide
Ex: HCl + NaOH
This always results in the creation of water and spectator ions
Strong acid + strong base
The strong acid dissociates completely and donates a proton to the weak base with the product being the conjugate acid
HCl +NH3
Weak acid + strong base
Strong base accepts protons from weak acid. Creates conjugate base of weak acid and water
HC2H3O2 + NaOH
Weak acid + weak base
Simple proton transfer
HC2H3O3 + NH3
Buffers
A buffer is a solution with a stable pH. Adding an acid, base, or water to a buffer solution will not greatly affect the pH.
A buffer is created by adding a significant amount of weak acid or base along with it’s conjugate as salt. This combination will not neutralize
NaC2H3O2 and HC2H3O2 added together will create a buffer solution.
If strong acid was added to this buffer solution it would create more acetic acid
If a strong base was added it would create more of the acetate ion
More concentration of the weak acid/base and its conjugate will create a stronger buffer solution
Henderson-Hasselbach equation can calculate exact pH of a buffer
pH = pKa + log([A-]/[HA])
[HA] = molar concentration of undissociated weak acid
[A-] is molar concentration of conjugate base
pOH = pKb + log([HB+]/[B])
[B] is molar concentration of weak base
[HB] is molar concentration of weak acid
When choosing an acid for a buffer solution, pick one with a pKa that is close to the desired pH.
A buffer cannot be created through a strong acid and its conjugate because it would dissociate completely. Same is said for bases.
Indicators
Indicators are weak acids that change colors based on pH change
The change/shift in reaction based on the ions in the solution causes the indicator to change color from bonding/dissociating
At the point of the color change, pKa = pH
Titration
Neutralization reactions usually occur as titrations
The pH increases slowly but then sharply after the equivalencepoint is reached, or when just enough base was added to neutralize the acid.
At the center of the buffer region is the half-equivalence point, where just enough base was added to turn half of the acid to its conjugate base
Unit Nine: Applications of Thermodynamics
Entropy
Entropy, S, is the amount of disorder or chaos in a system. More disorder, greater S value.
Standard entropy is S° and measured at 25 celsius
Standard entropy change ∆S° is measure at the end of a reaction
∆S° = (sum of ∆S° products) - (sum of ∆S° reactants)
If a reaction goes from less moles to more moles (such as 2 moles on the reactant side to 3 moles on the product side) there is more disorder and a positive ∆S
If a reaction goes from a gas to liquid, liquid to solid, or gas to solid, the reaction has a negative ∆S
If bonds are broken and phase change becomes more disordered, the ∆S is positive
Gibbs Free Energy
∆G is Gibbs Free Energy which determines if a process is thermodynamically favored or unfavored, also known as spontaneous or nonspontaneous
Free Energy Change
Standard free energy change, ∆G°, is calculated the same as ∆S°
∆G° = (sum of ∆G° products) - (sum of ∆G° reactants)
For a reaction,
If ∆G is negative, it is TFP (thermodynamically favored process)
If ∆G is positive, it is not TFP
If ∆G is 0, it is at equilibrium
∆G, ∆H, and ∆S
TFP must result in decreasing enthalpy, increasing entropy, or both
∆G° = ∆H° - T∆S°
T = temperature in Kelvin
∆S° is usually given in j/mol*K and must be converted to kj/mol*K
Gibbs Free Energy is usually kj/mol*K
∆H | ∆S | T | ∆G | Favorability |
|---|---|---|---|---|
- | + | LowHigh | -- | Always TFP |
+ | - | LowHigh | ++ | Never TFP |
+ | + | Low High | +- | Not TFPTFP |
- | - | Low High | -+ | TFPNot TFP |
Standard Free Energy Change and the Equilibrium Constant
Gibbs free energy can be calculated if equilibrium constant is known
∆G° = -RT(ln K)
R = gas constant (8.31 j/mol*k)
T = kelvin temperature
K = equilibrium constant
If ∆G° is negative, K is greater than 1, the products are favored at equilibrium
If ∆G° is positive, K must be less than 1, the reactants are favored at equilibrium
Reduction Potentials
Every half reaction has electric potential. Potentials are given as reduction half-reactions. If the reaction is reversed, flip the sign to get the oxidation potential
Galvanic Cells
Galvanic cells (voltaic cell) use favored redox reactions to generate current
Two half-reactions take place in separate chambers and the electrons from the oxidation pass to the reduction reaction which creates the current
Current is defined as the flow of electrons from one place to another
Oxidation takes place at the anode electrode and reduction takes place at the cathode electrode
The salt bridge keeps electrical neutrality. Without the salt bridge the voltage would be zero. The potassium ion flows to the cathode and the chlorine flows to the anode.
The cell voltage is equal to the total redox reaction voltage.
Non-Standard Conditions
Reduction potentials are give at standard conditions, 25 celsius, 1 atm, and 1 M
Voltaic cells are very favored with equilibrium constant greater than 1. If the Q = K however, the voltage would drop to ero.
If the reaction quotient increased it would become close to the equilibrium constant and the voltage would decrease.
Electrolytic Cells
Electrolytic cells use outside voltage sources to power unfavored redox reactions and mainly occur in aqueous solutions.
The sign of total cell potential is always negative
Electroplating
Electrolytic cells are used for electroplating.
I = (q/t)
I = Current (amperes, A)
q = charge (coulombs, C)
t = time (second, s)
Moles of electrons = (coulombs/ 96,500 coulombs per mol)
Voltage and Favorability
Redox is favored if the potetial has a positive value. reaction potential can be calculate gibb’s free energy
∆G° = -nFE°
n = number of moles of electrons exchanged in the reaction
F = Faraday’s constant. 96,500 coulombs/mol
E° = standard reaction potential (V)
If E° is positive, ∆G° is negative and is TFP
If E° is negative, ∆G° is positive and not TFP