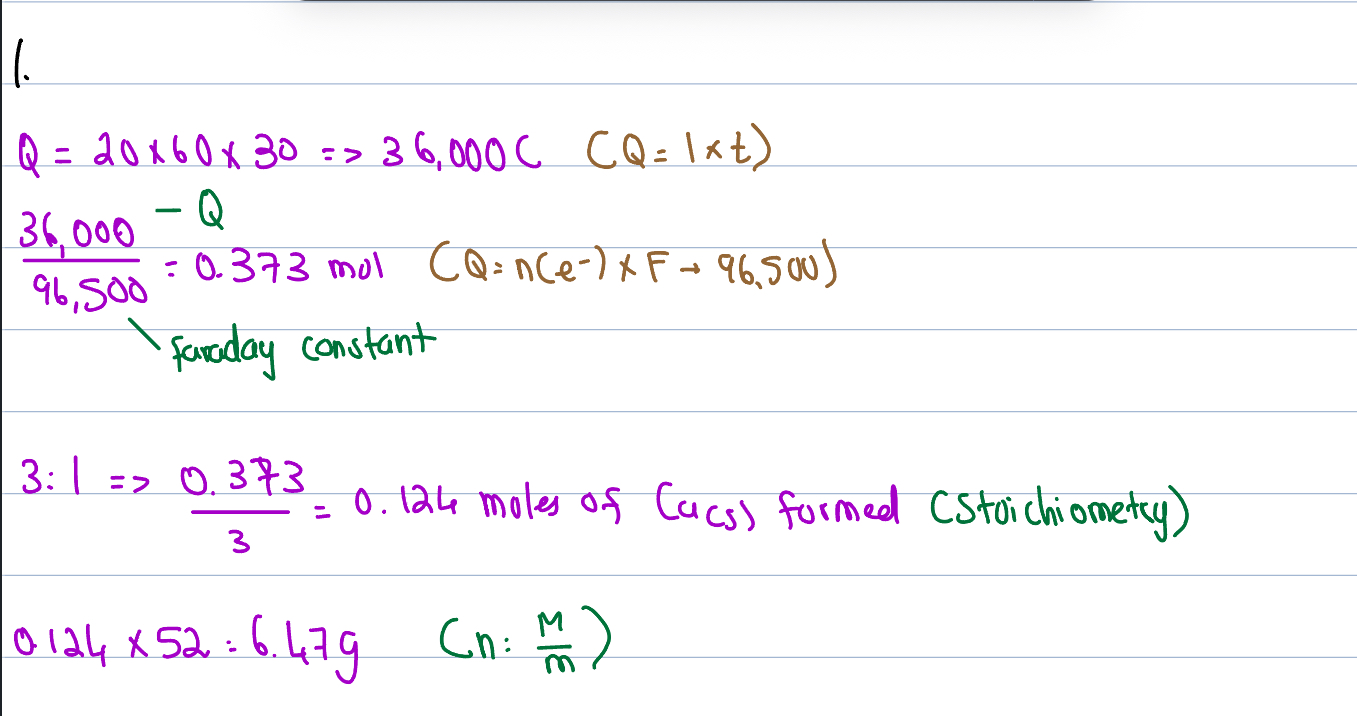Electrolysis - Chapter 8
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
Electrolysis
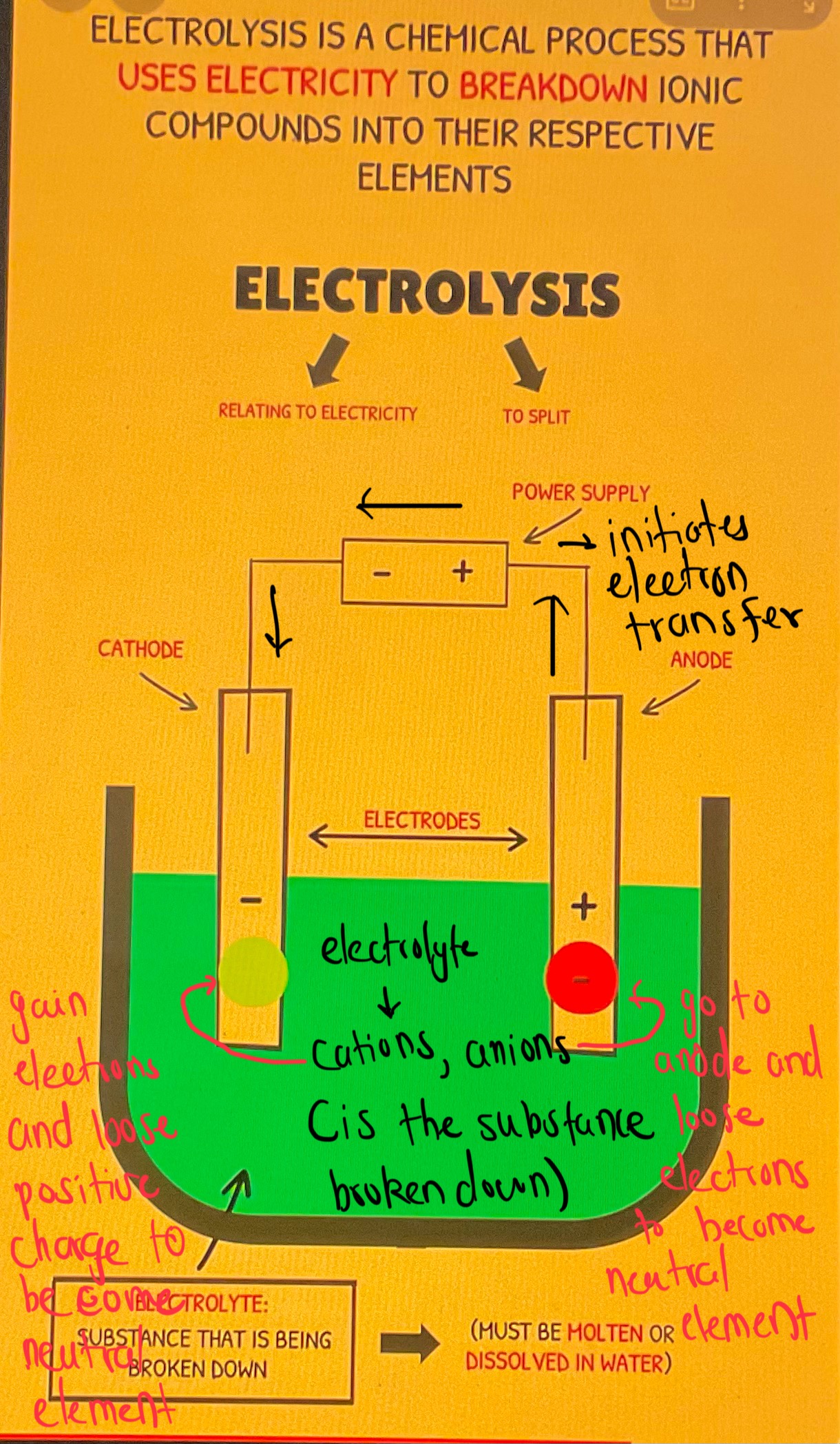
Uses electricity to break down a compound into its simple elements
Uses electrical energy to form chemical energy (reverse of Galvanic Cells)
How Electrolysis Works
The electrolyte is compound which is to be broken apart
The battery initiates electron movement
As the anions and cations break apart to the electrodes, distinct compounds are formed
Visual Explanations
What Electrodes Are Used?
Inert Electrodes are used (carbon, platinum, graphite)
So that the electrodes themselves do not interact with the reaction
Difference between spontaneous and non-spontaneous
Spontaneous doesn’t require external input for a reaction
Non-spontaneous requires external power stimulus for the reaction
Why do products need to be kept apart in electrolytic cell
If the products contact one another, they can form back to being reactants
When there are multiple reactants in the cell
Electrodes themselves, water and reactanst could all be reactants
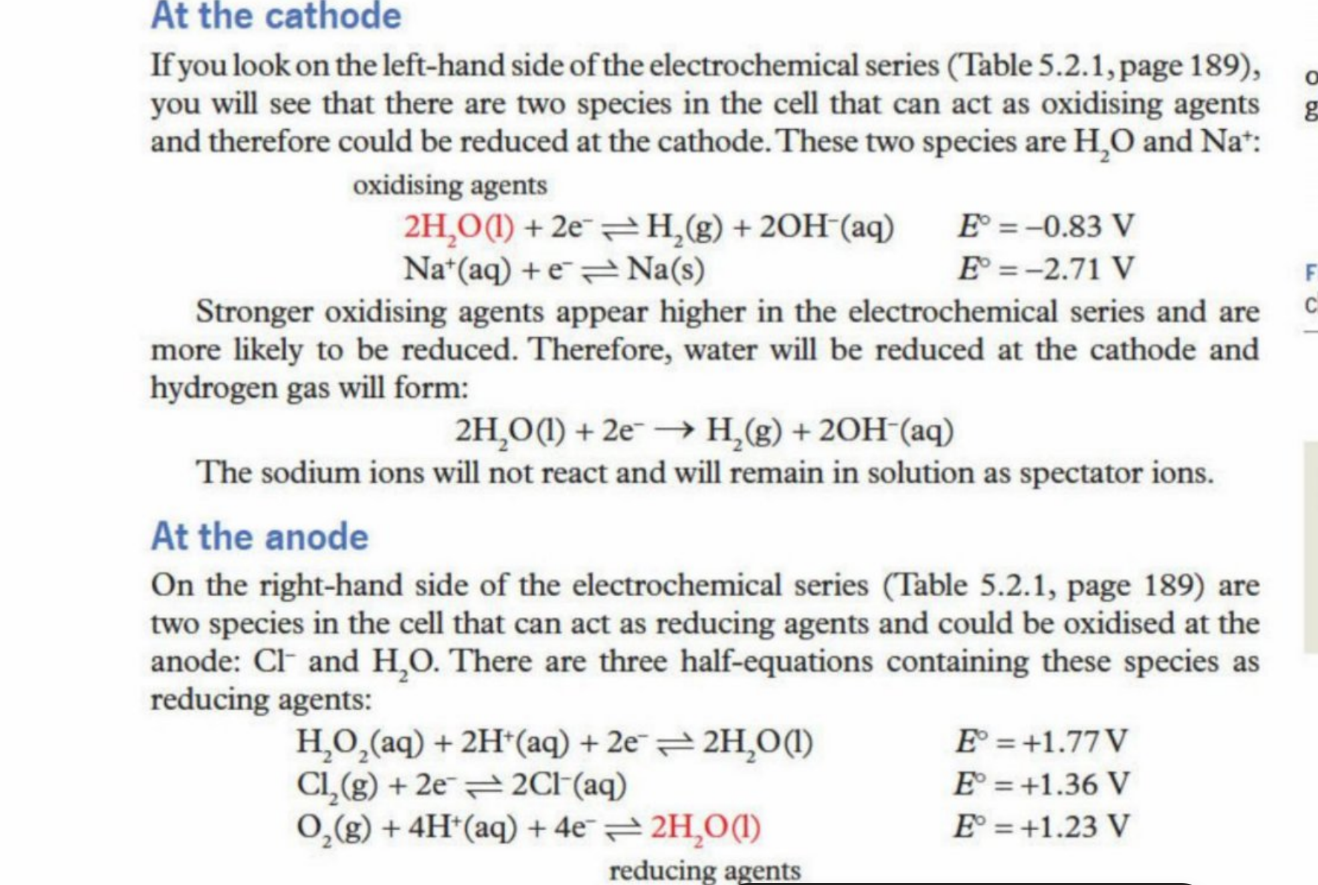
Strongest oxidising agent (one with highest emf value - at cathode)
Strongest reducing agent (one with lowest emf value - at anode)
Example
Inert Vs Reactive Electrodes
Inert electrodes - don’t participate in reaction
Reactive electrodes - are reactants in reaction (use electrochemical series to identify possible reactants)
Galvanic Vs Electrolytic
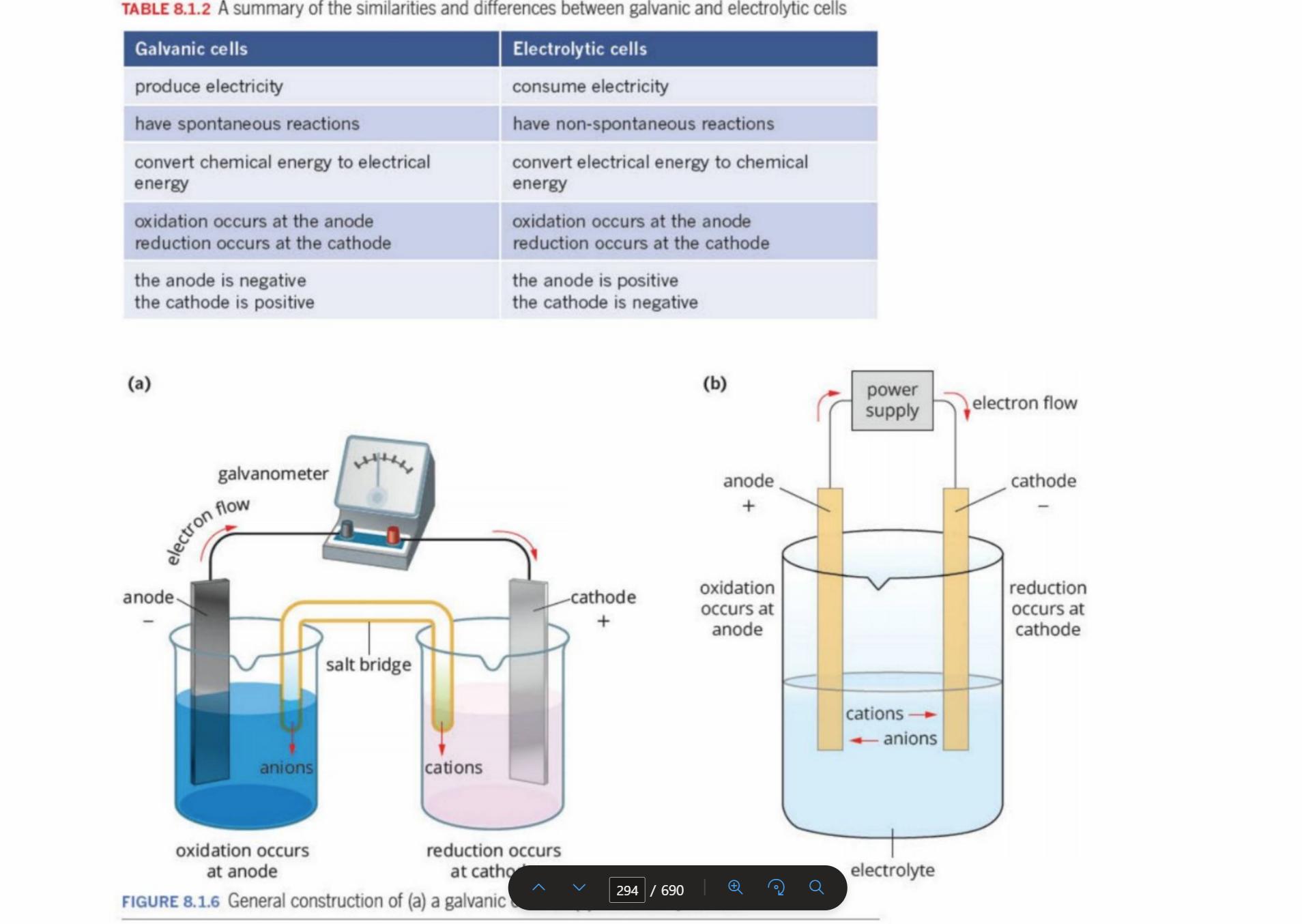
Why Anode In Electrolytic Cell Is Positive
Electrons are being stripped/deprived contunally due to the power supply - hence turns into a positive region
“electron deficient”
Why Anode In Galvanic Cell Is Negative
Electrons are being continually formed and are accumulated, despite the electron flow occuring
“Electrons are born there”
Why Are There Gases As Products
1) Gases form in voltaic (galvanic) cells when oxidation or reduction involves ions or molecules that become neutral gas molecules after gaining or losing electrons.
2) Gases form in voltaic cells when oxidation or reduction converts aqueous ions into neutral molecules, which are unstable in solution and therefore evolve as gases.
Down Cell
An electrolysis cell that seperates NaCl into Na and Cl in commercial quantities
Is done through molten NaCl
Down Cell (Image)
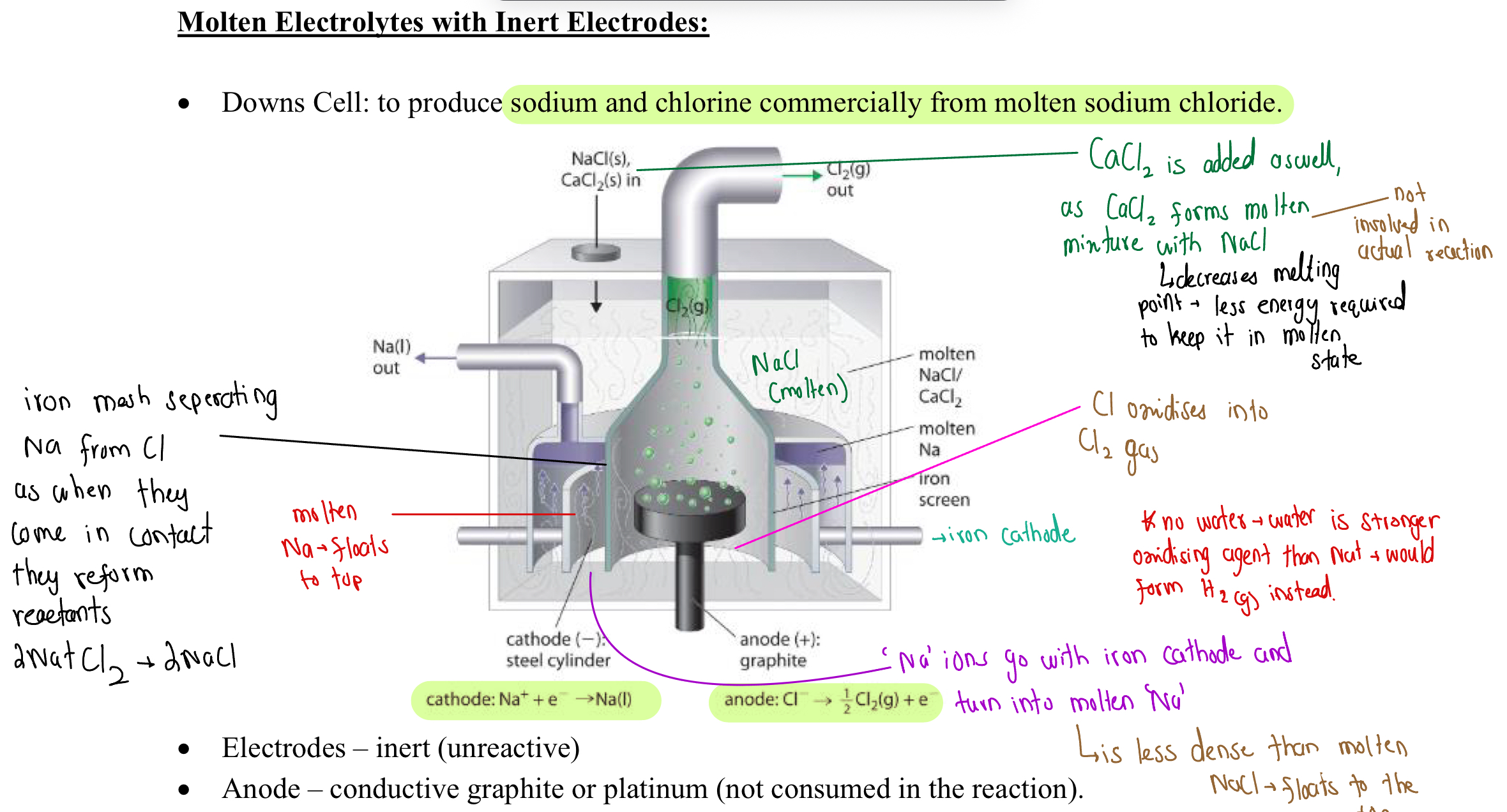
Why Water IS Not In Down Cell
Water is a stronger oxidizing agent than Na ions - would create H2 gas instead of Na (l) at the anode
Na ions also react very violently with H20
Why Is Iron Inert As An Electrode (Unclear)
Electrons are continually pumped through the electrode, preventing it from oxidizing
Why Are Aqueous Electrolytes More Preferable to Molten Electrolytes
It is more cost-effective
Energy to keep molten electrolyte is very high - keeping it in aqeous state requires less energy and cost
Membrane Cell
Used to create hydrogen gas, chlorine gas and NaOH
Membrane Cell (Visual Representation)
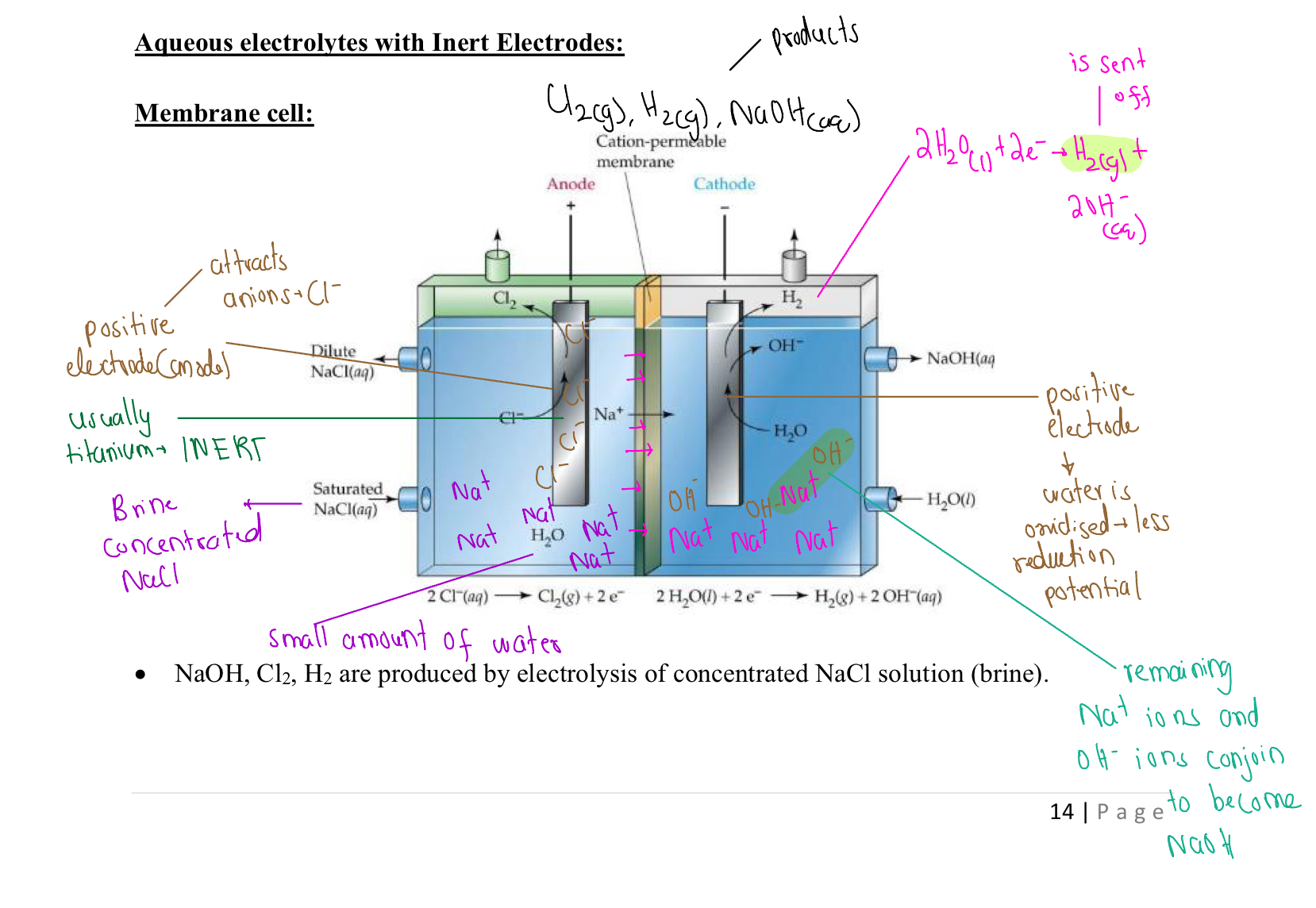
Benefits Of Membrane Cell
The NaOH is seperated from the NaCl
The aqeuous electrolyte reduces the need for intensive heat and, reduces costs on energy
Why Metals in cathode don’t corrode
Oxidation = corrosion
In cathode, excess of electrons are being fed in, preventing the metal oxidising and loosing electrons - there is a continual supply of electrons
Electrolytic Production Of Aluminium
Done through Hall-Heroult cell, and doen with molten aluminium
Visual Of Hall-Heroult Cell
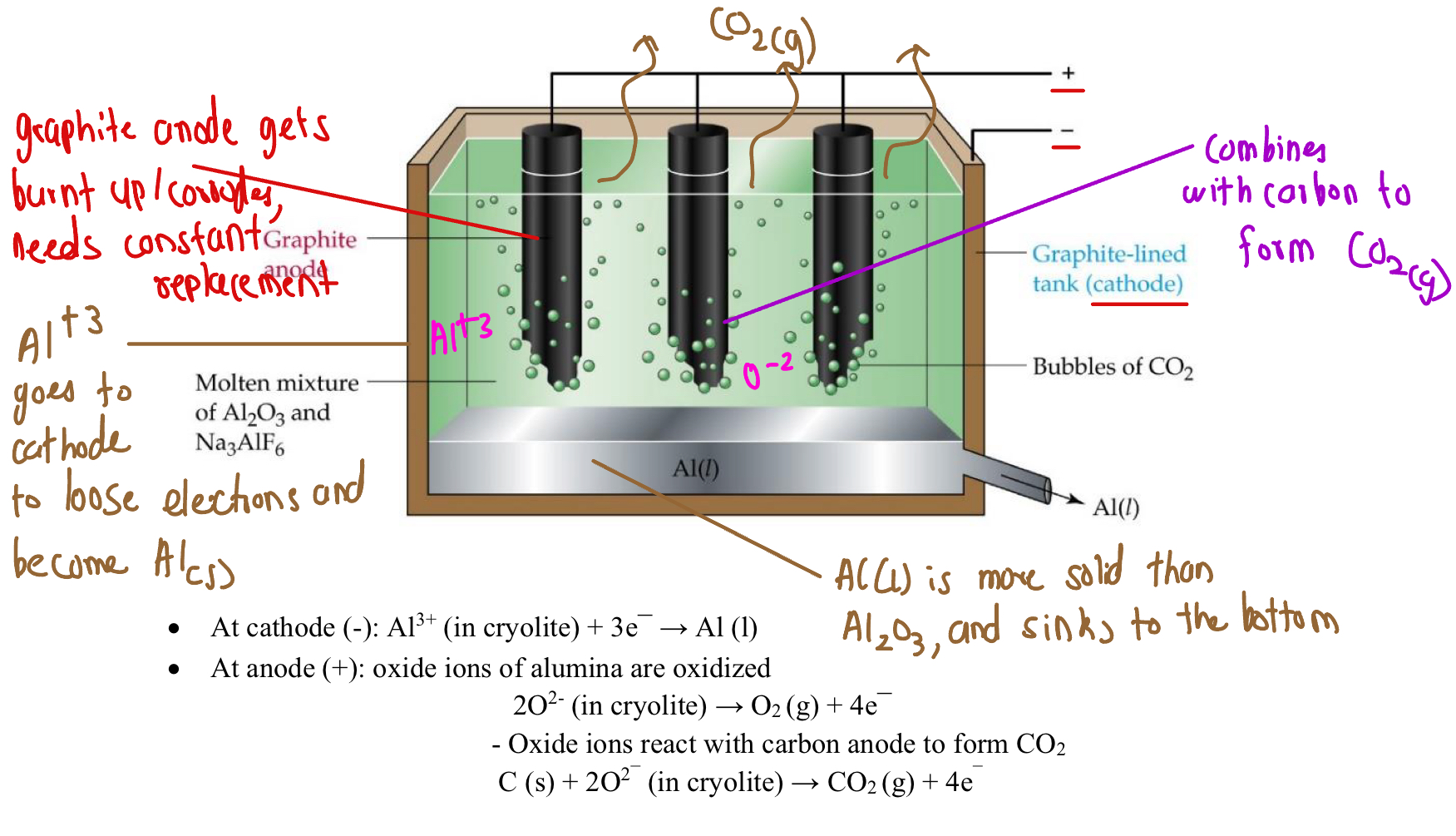
Why is Cryolite (Na3Al2O3) used
It is used as a solvent to pure alumiunm, to reduce the melting point
Allows to conduct electricity better
Reduces overall melting point (2072 - 1000 degrees)
Prevents excessive anode wear, as lower temperature extends lifespan
Equation at Anode (Graphite) - 2 Step Process
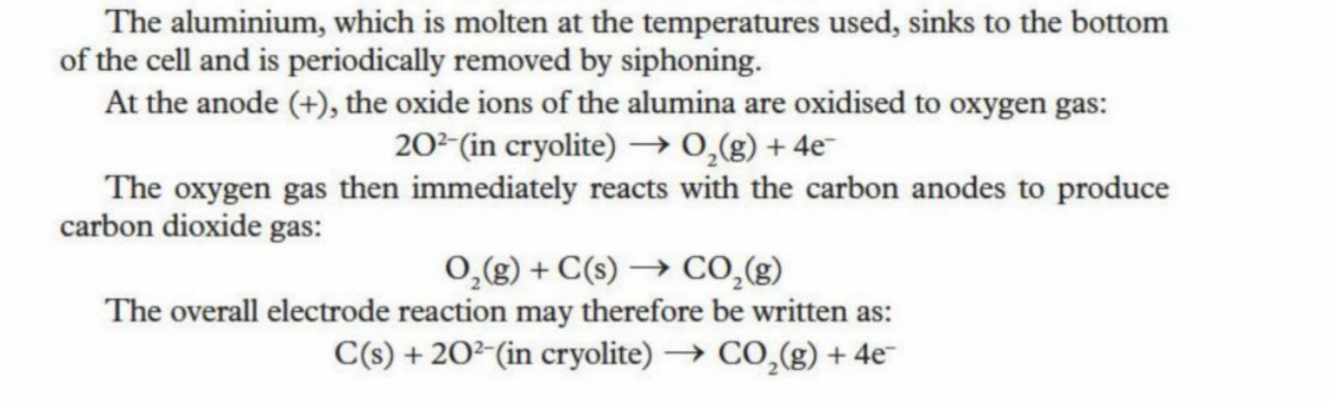
Two Step Process At Anode
The oxygen is oxidised to oxygen gas
The oxygen gas then immediately reacts with the carbon anode, producing oxygen gas
Reaction at cathode
Is lined with graphite
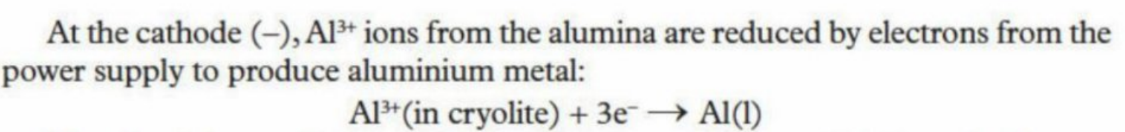
Overall Reaction

Why this current model is not ethical
Intensive energy requirement
Constant replacements of the graphite anode (is reactive - participates in the reaction) - very expensive
Releases CO2
Ways to shift to another model
Using inert electrodes (platinum, gold) - not feasible on a global scale
Alcoa and Rio Tinto Idealogy - using proprietayr electrodes - makes O2 gas instead of CO2
Can reduce costs, improve aluminum production and be done sustainably
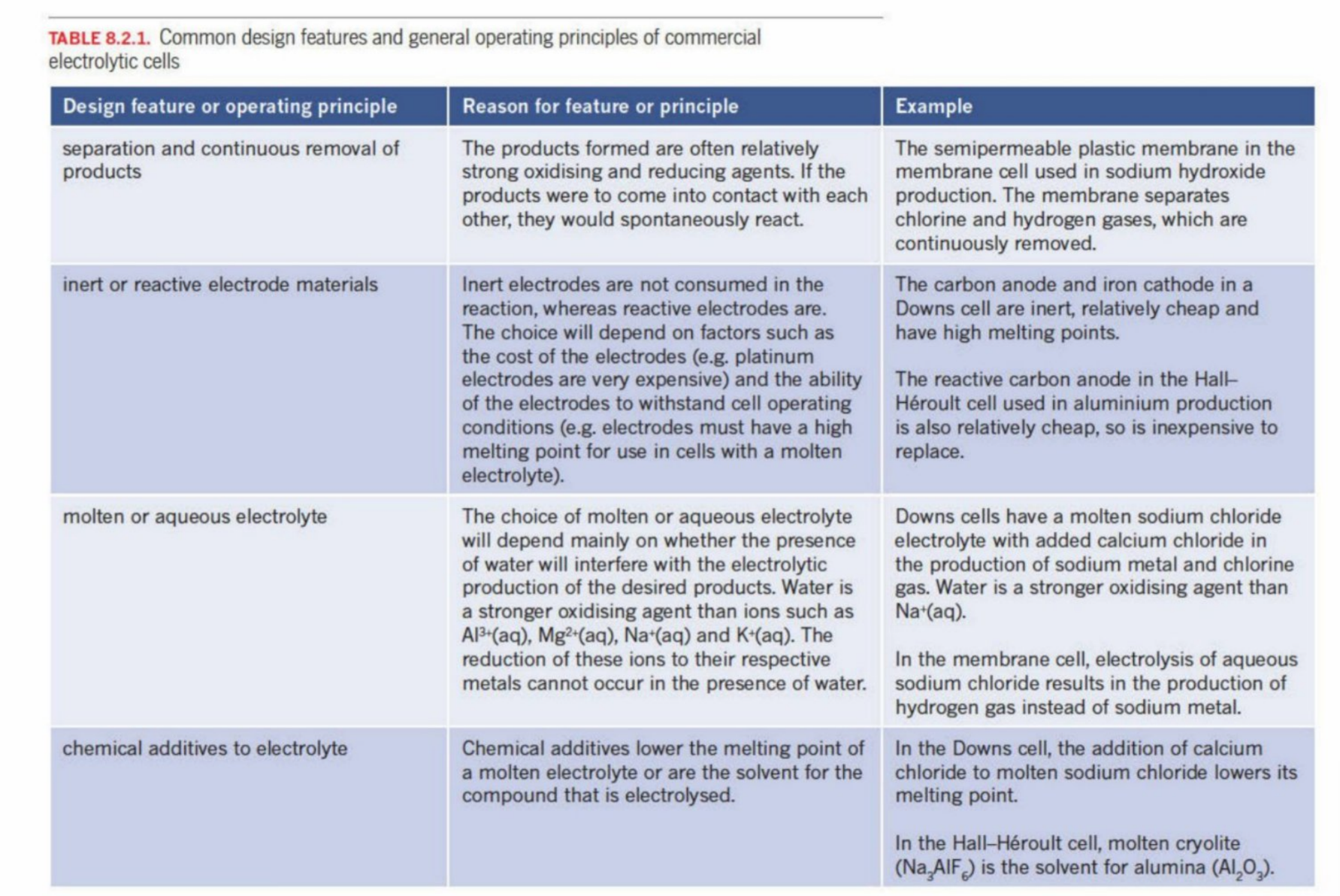
Common Features & Reasoning In Electrolytic Cells
Electroplating
Anotehr metal (thin layer) is cast upon another surface to prevent corrosion or oxidation of the metal underneath
Can have a stable metal or a strong reductant, used as sacrificial protection
How Does It Work
Cathode is the item needing to be plated - negative electrode
From the anode, ions are flowing out, keeping the electrolyte’s concentration of ions balanced
The ions are attracted to the negative electrode and react with the electrons to form a solid element
They then coat around the cathode (the item needing to be plated)
Visual Representation
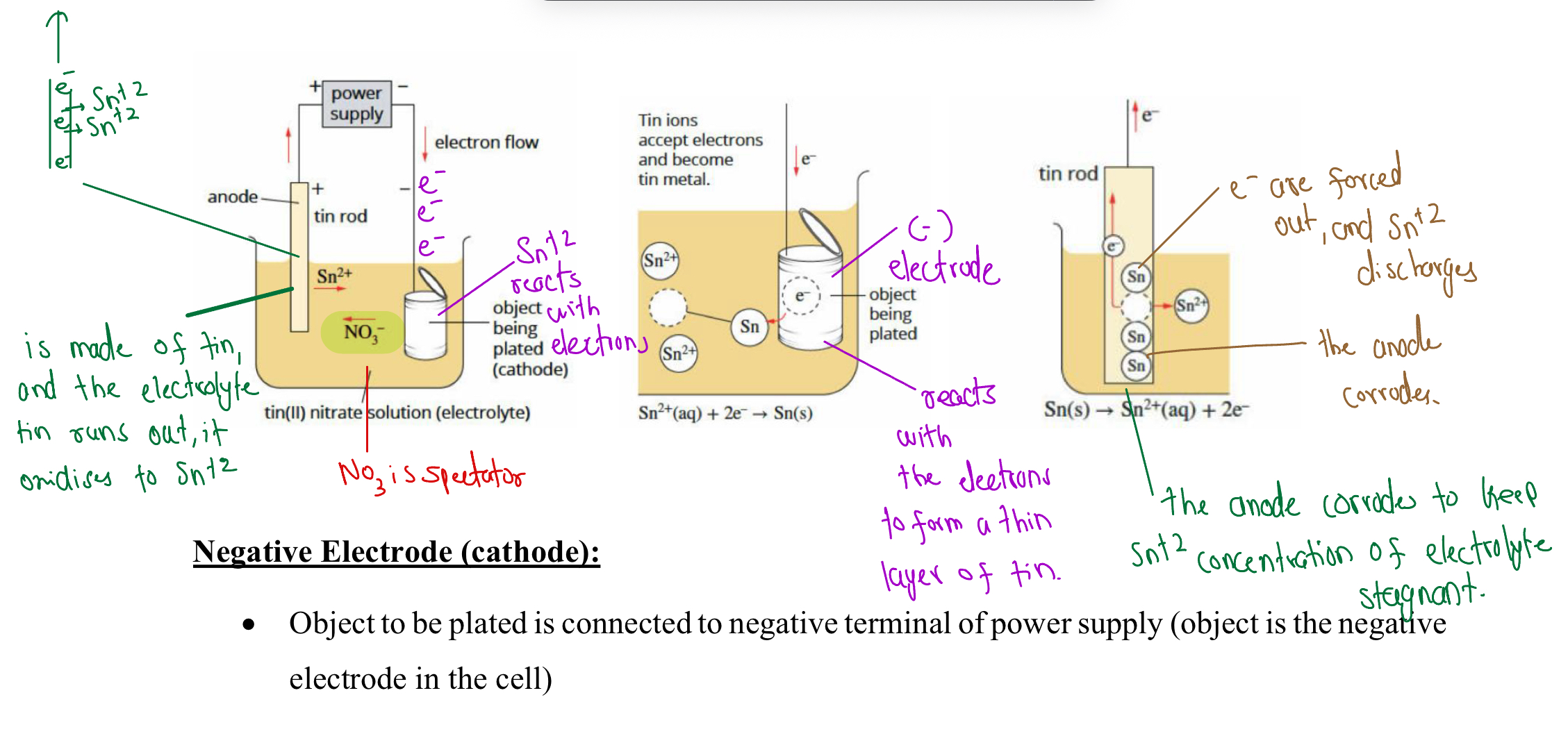
Example with tin (at anode)
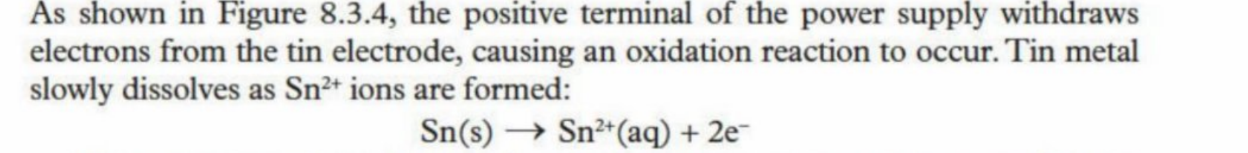
Example with tin (at cathode)
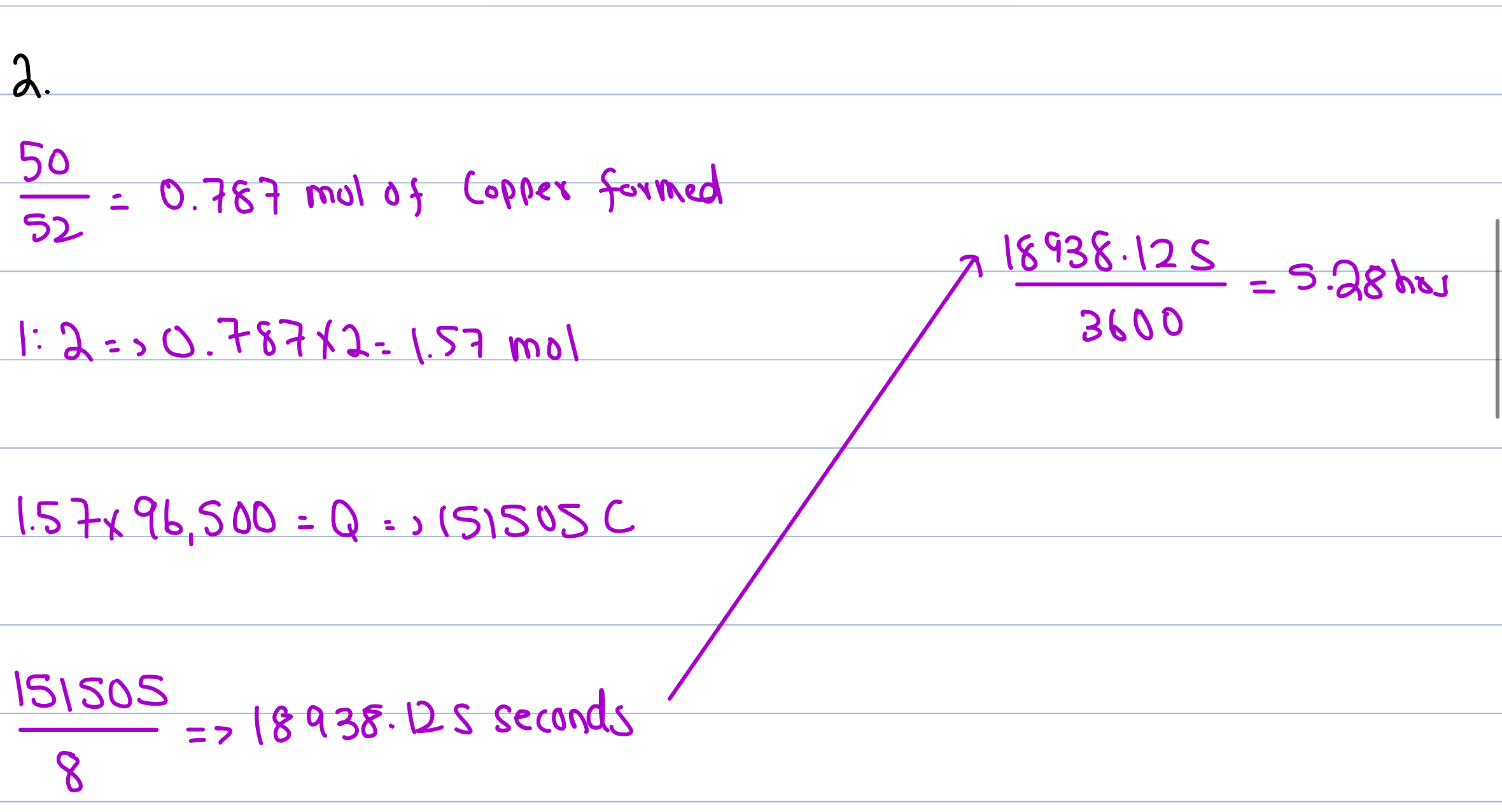
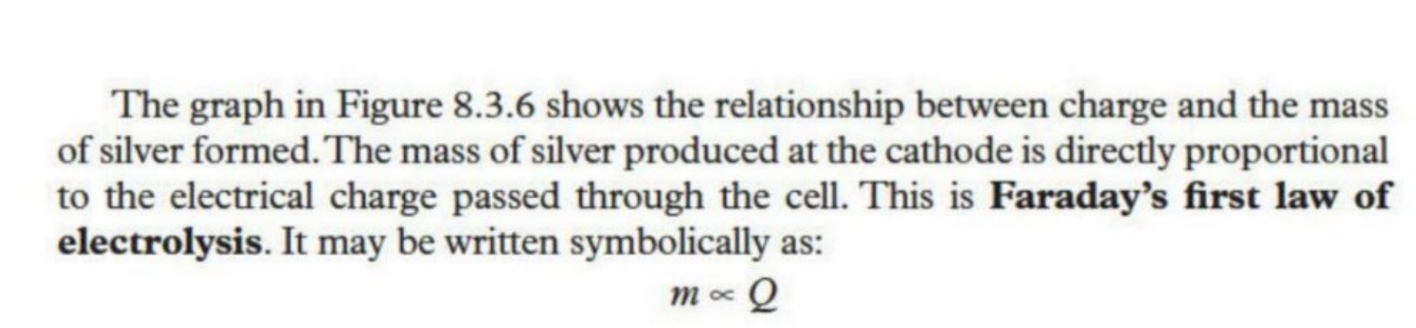
Faraday’s First Law
The mass of metal produced at the cathode is directly proportional to the amount of electric charge is passed through (Q - Coulombs)
Relationship for charge
Q (Coloumbs) = current (amps) * time (seconds)
Faraday’s Second Law
Though Mass is directly proportional to Q, it is done at different rates per different metals
The no. of moles of electrons required to create the mass is measured
Requiring less electrons = more yield, (sharper gradient)
e.g. Ag only needs 1 mol of electrons - has sharpest gradient
Charge of 1 mol of electrons
1 mol of e- = 96,500C (coloumbs)
Relationship between Q & C (2nd Law)
Q = n(e-) * F
e.g. Copper:
Cu+2 + 2e- ——→ Cu(s) = 2×96,500 → 193,000 Coloumbs