Chapter 9: Visualizing Cells
Looking at cells in the light microscope
Light Microscopy:
Light microscopy is a widely used technique for observing and studying cells and their structures.
It uses visible light to illuminate the sample and magnifying lenses to generate an enlarged image.
Light microscopes are relatively simple to use, cost-effective, and provide valuable insights into cell structure and function.
Brightfield Microscopy:
Brightfield microscopy is the most common type of light microscopy.
It uses transmitted white light to illuminate the sample, which results in a dark specimen against a bright background.
It is suitable for observing stained or naturally pigmented cells and tissues.
Contrast Techniques:
Various contrast-enhancing techniques are used in light microscopy to improve the visibility of cellular structures.
Staining with dyes or fluorescent probes highlights specific components within the cell, such as nuclei, cytoplasm, or organelles.
Phase contrast microscopy and differential interference contrast (DIC) microscopy enhance the contrast by exploiting differences in refractive index within the specimen.
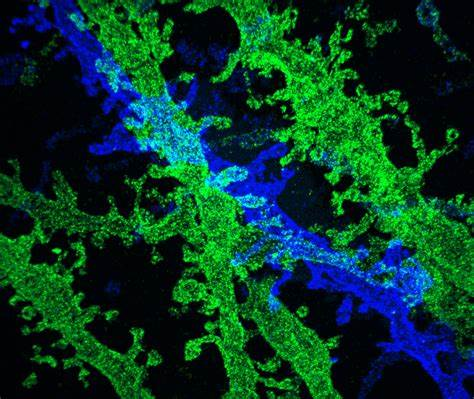
Fluorescence Microscopy:
Fluorescence microscopy utilizes fluorescent molecules to visualize specific structures or molecules within cells.
Fluorescent dyes, antibodies, or genetically encoded fluorescent proteins are used to label the target molecules.
Excitation of the fluorophore with a specific wavelength of light causes it to emit light at a longer wavelength, which is detected by the microscope.
Fluorescence microscopy enables the visualization of dynamic processes, protein localization, and molecular interactions within cells.
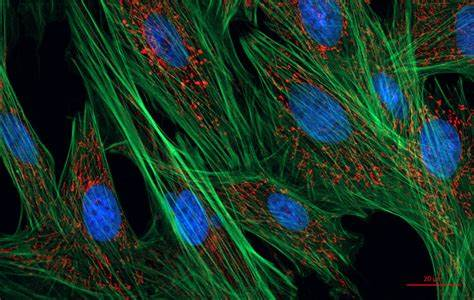
Confocal Microscopy:
Confocal microscopy is a specialized form of fluorescence microscopy that provides optical sectioning and improved resolution.
It uses a pinhole aperture to eliminate out-of-focus light, allowing for the capture of sharp images at different depths within the specimen.
Confocal microscopy is useful for 3D reconstruction of cellular structures and live-cell imaging.
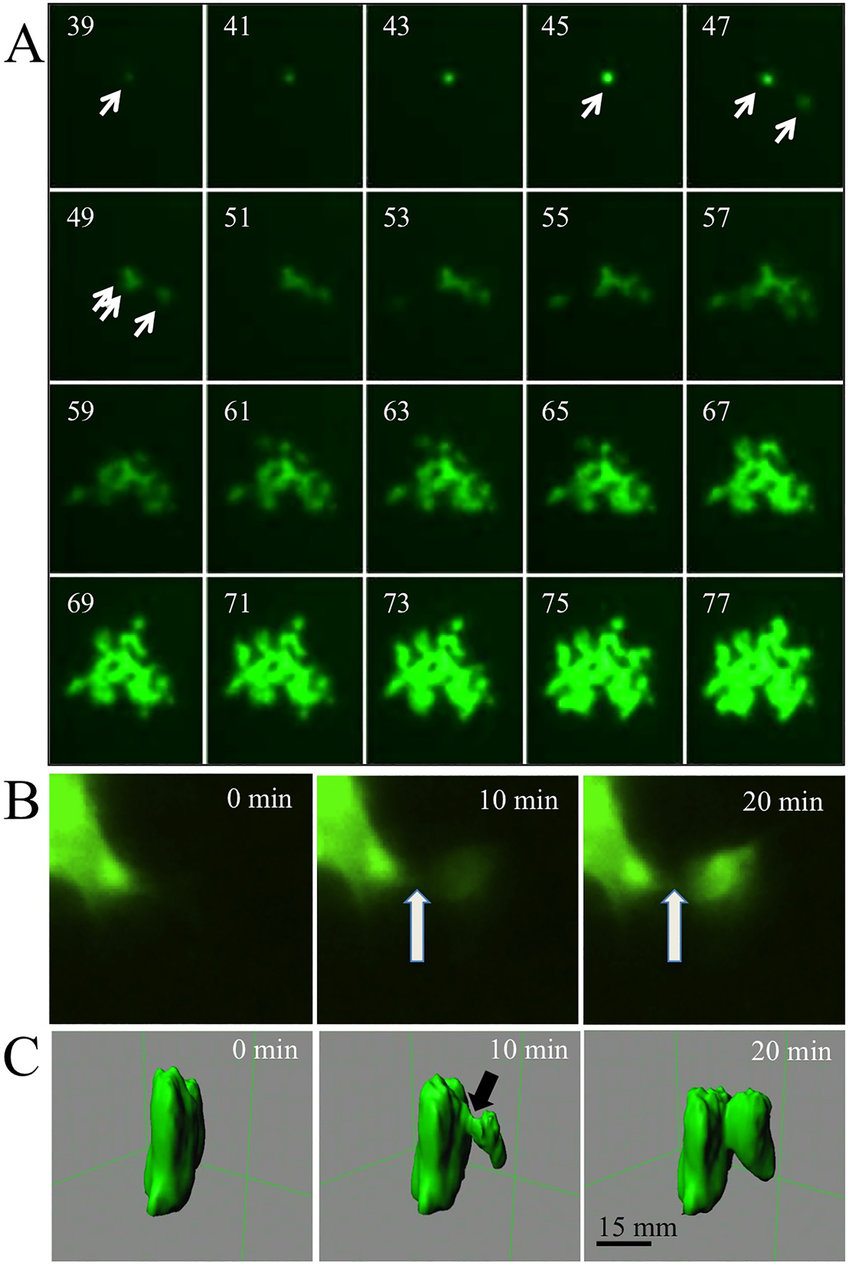
Time-Lapse Microscopy:
Time-lapse microscopy captures images of cells or tissues at regular intervals over a defined period.
It allows the observation of dynamic processes, such as cell division, cell migration, or intracellular transport.
Time-lapse microscopy provides insights into cell behavior, growth, and response to stimuli.
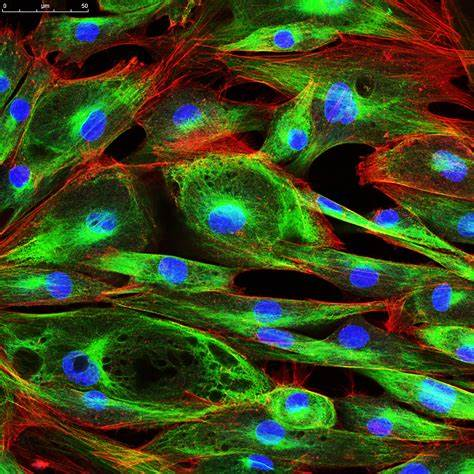
Immunofluorescence:
Immunofluorescence combines the specificity of antibodies with fluorescence microscopy.
It involves the labeling of specific proteins or molecules with fluorescently labeled antibodies.
Immunofluorescence enables the visualization of protein localization, interaction networks, and cellular structures.
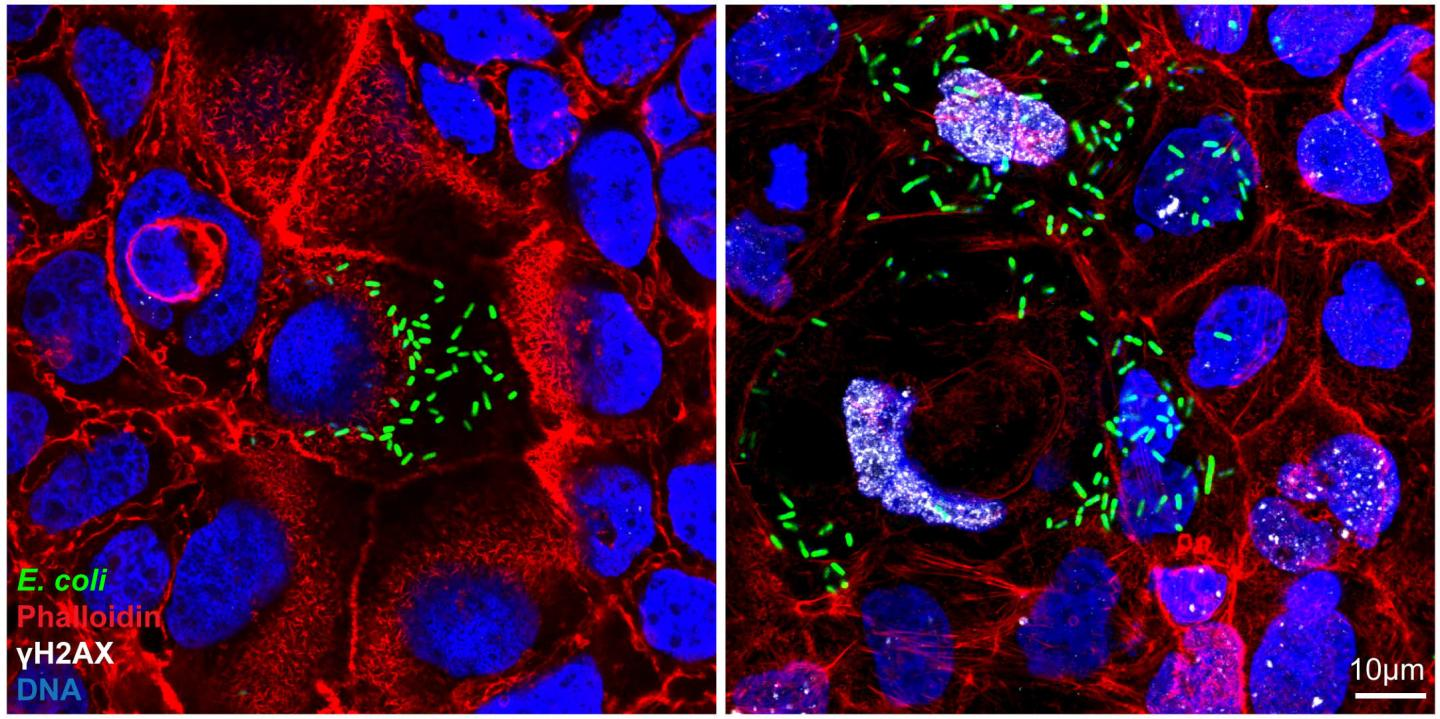
Live-Cell Imaging:
Live-cell imaging allows the observation of living cells over time.
It requires specialized microscope setups with environmental control to maintain optimal temperature, humidity, and CO2 levels.
Live-cell imaging provides insights into cellular dynamics, cell behavior, and response to stimuli.
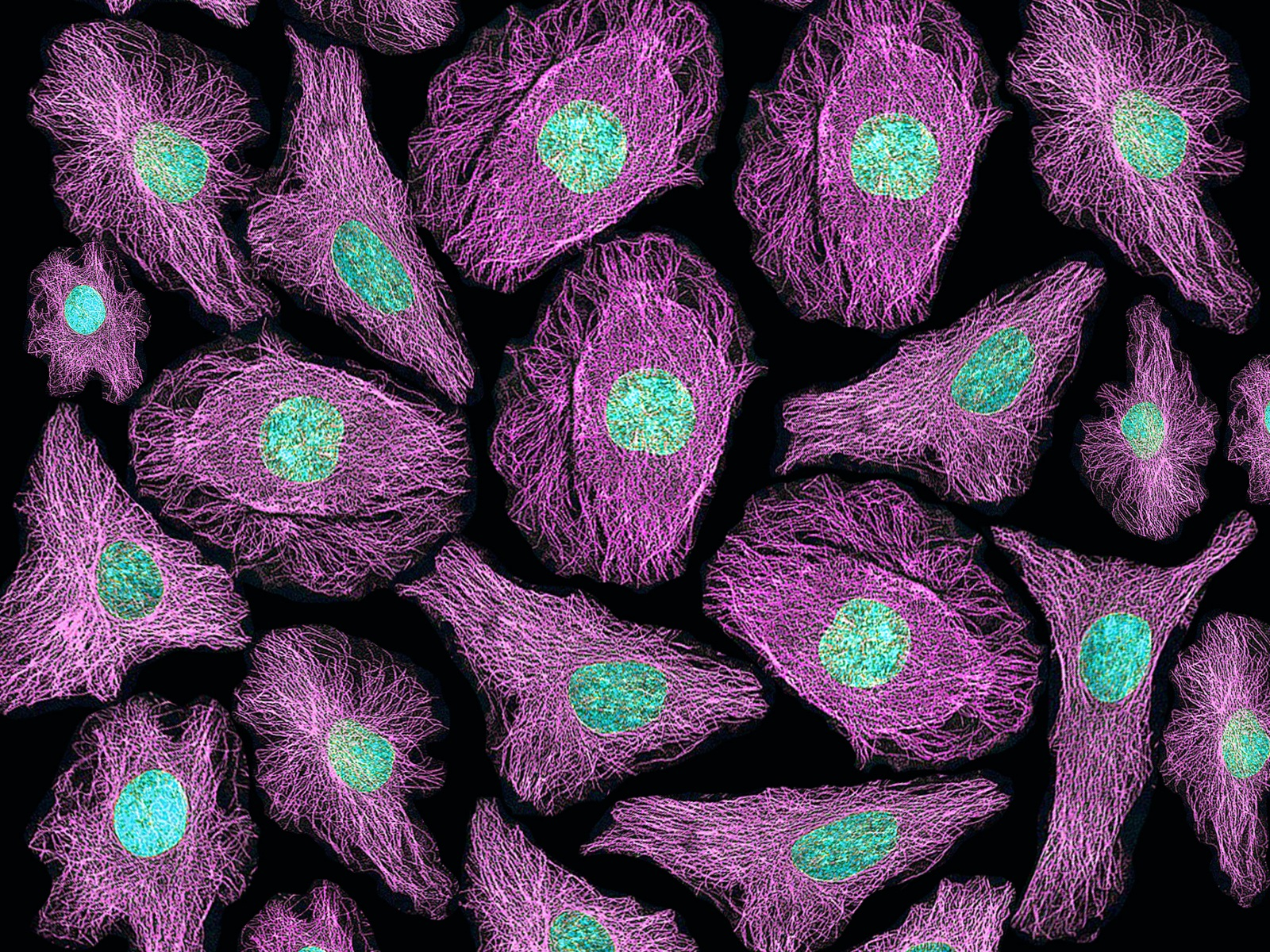
Resolution and Magnification:
- The resolution of a light microscope determines the level of detail that can be observed.
- Resolution is influenced by factors such as the wavelength of light, numerical aperture of the objective lens, and the quality of the microscope.
- Higher magnification allows for the visualization of smaller cellular structures, but it is limited by the resolution of the microscope.
Looking at cells and molecules in the electron microscope
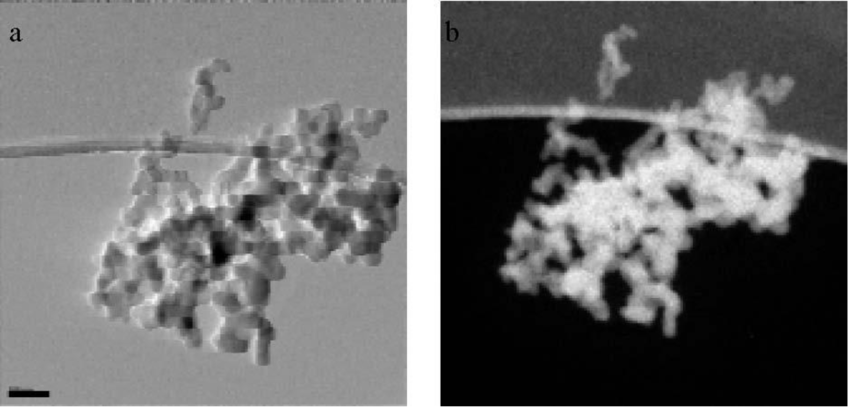
Electron Microscopy:
Electron microscopy is a powerful imaging technique that uses a beam of electrons instead of light to visualize cells and molecules at high resolution.
It provides detailed information about cell ultrastructure and allows for the visualization of subcellular organelles and molecular complexes.
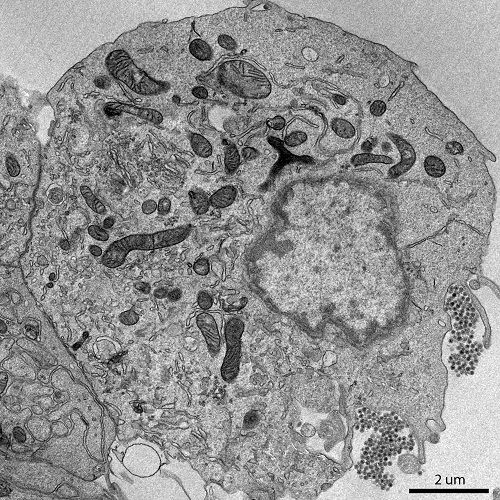
Transmission Electron Microscopy (TEM):
Transmission electron microscopy involves passing a beam of electrons through an ultrathin sample to generate an image.
It provides high-resolution images with magnifications up to several hundred thousand times.
TEM is used to study the internal structures of cells, such as organelles, membranes, and cellular components.
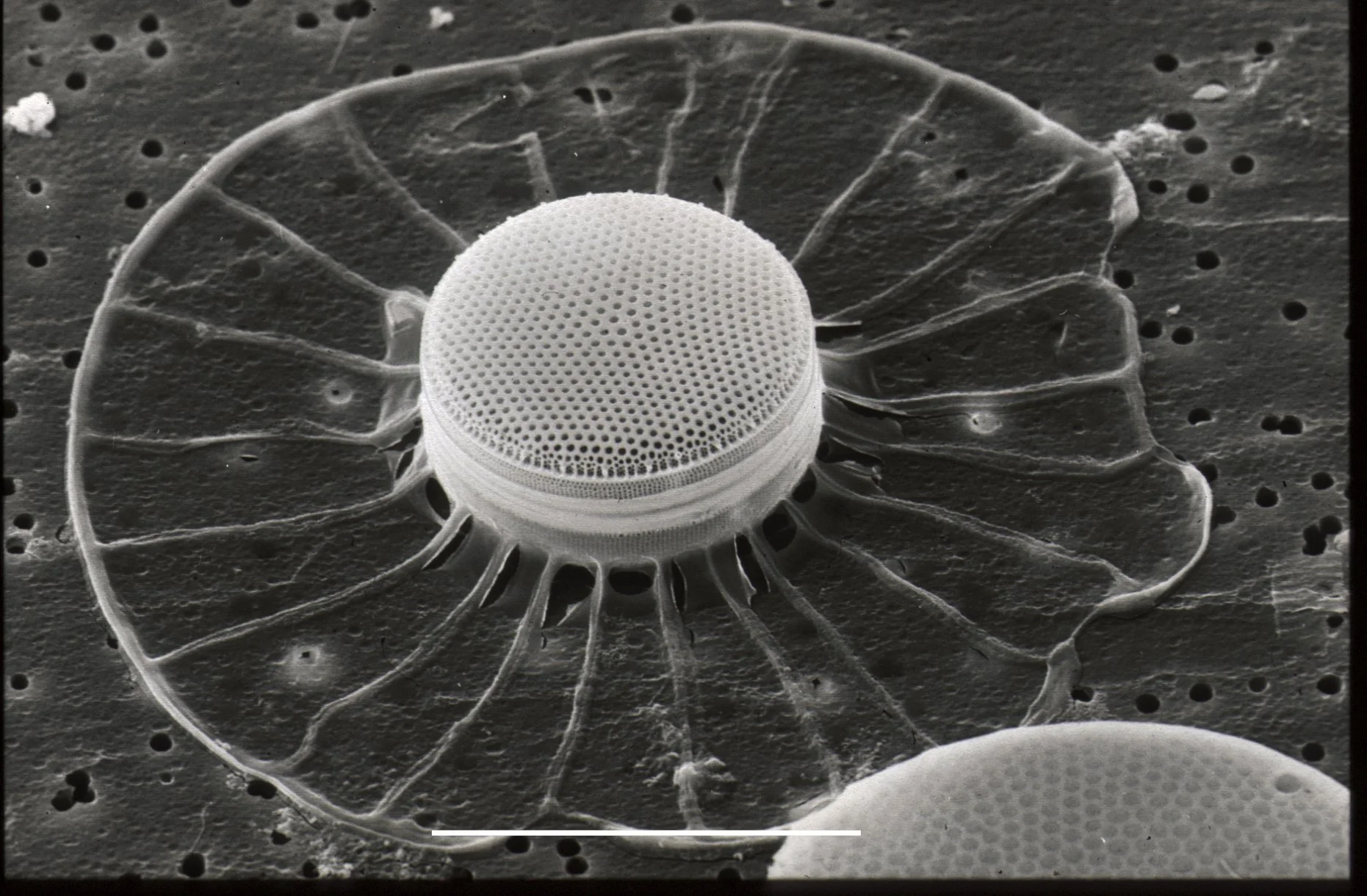
Scanning Electron Microscopy (SEM):
Scanning electron microscopy involves scanning a focused beam of electrons across the surface of a sample.
It provides high-resolution 3D images of the sample's surface morphology.
SEM is used to study the surface features of cells, tissues, and other specimens.
Sample Preparation:
- Preparing samples for electron microscopy requires careful processing to ensure proper visualization and preservation of cellular structures.
- For TEM, samples are typically fixed, dehydrated, embedded in resin, and sectioned into ultrathin slices.
- SEM samples are often fixed, dehydrated, and coated with a thin layer of conductive material (e.g., gold or platinum).
Contrast Techniques:
Electron microscopy uses various contrast techniques to enhance the visibility of cellular structures.
Staining with heavy metal salts, such as uranyl acetate or lead citrate, provides contrast by selectively binding to different cellular components.
Immunogold labeling utilizes gold nanoparticles conjugated to antibodies to localize specific molecules within the sample.
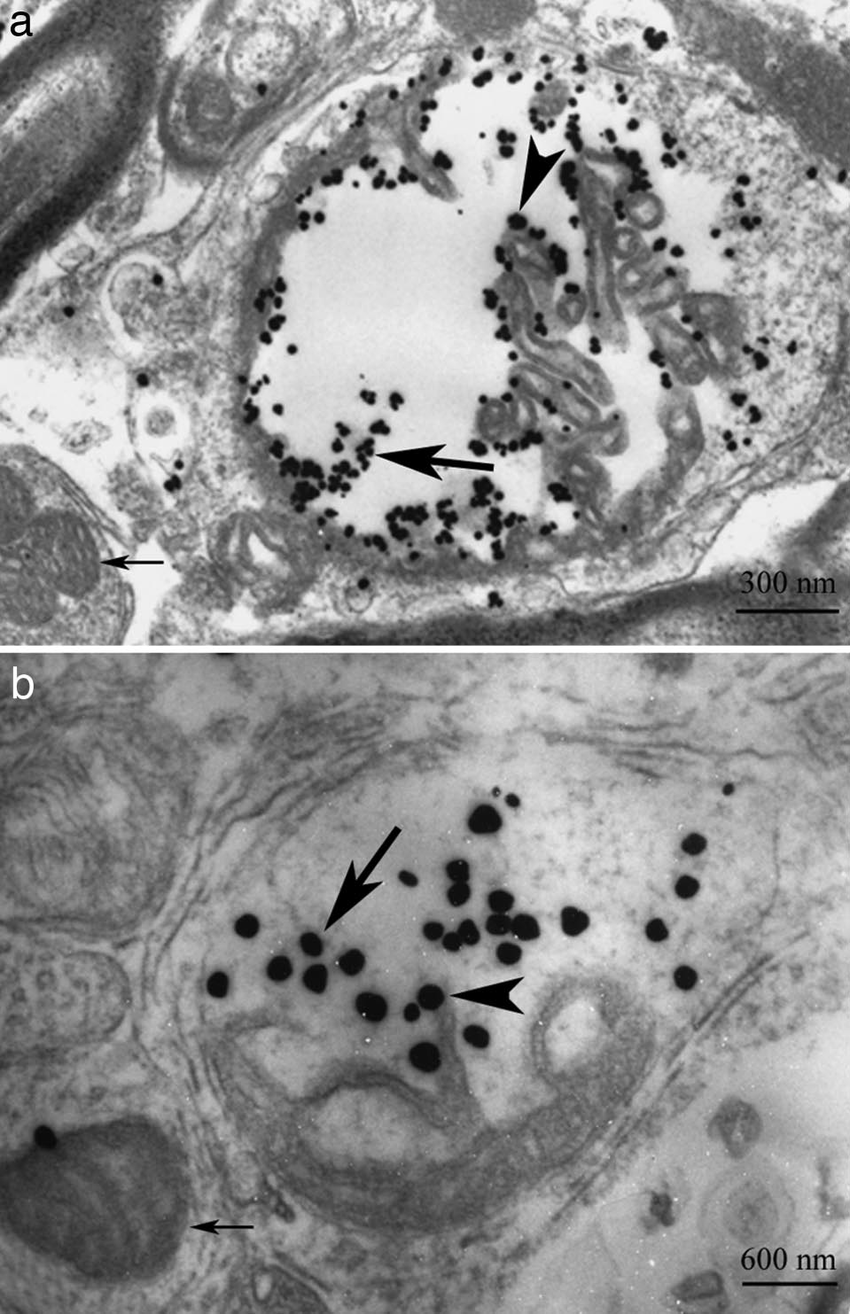
High-Resolution Imaging:
Electron microscopy provides high-resolution imaging, allowing for the visualization of cellular structures at the nanometer scale.
It reveals details such as the arrangement of macromolecules, membrane structures, and fine cellular features.
High-resolution imaging is essential for studying molecular interactions, protein complexes, and cellular organization.
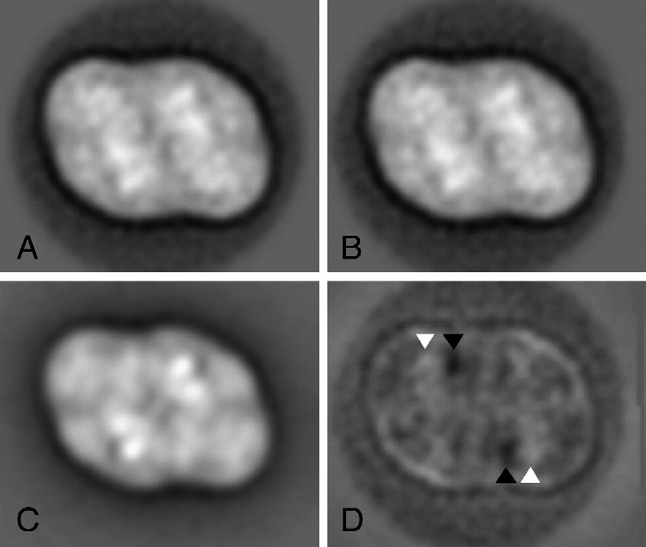
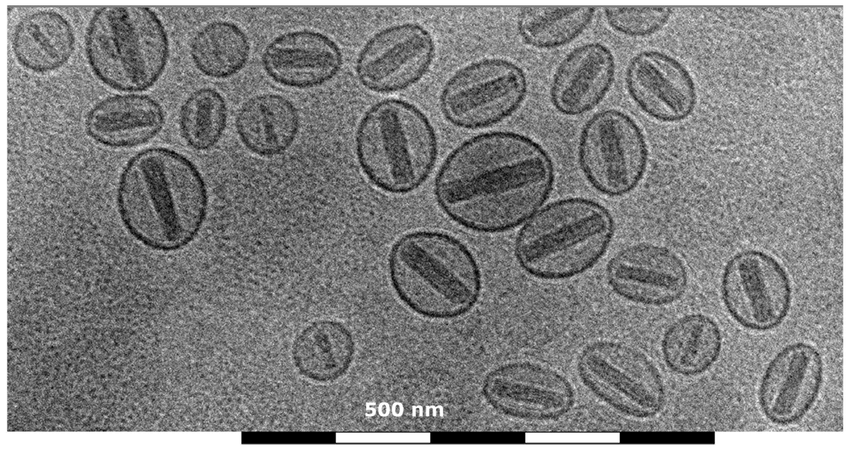
Cryo-Electron Microscopy (Cryo-EM):
Cryo-EM is a technique that allows for the imaging of samples in their near-native, frozen-hydrated state.
It involves rapidly freezing the sample to preserve its structure and then imaging it at cryogenic temperatures.
Cryo-EM has revolutionized structural biology by enabling the determination of high-resolution structures of macromolecules and complexes.
Electron Energy Loss Spectroscopy (EELS):
EELS is a technique that measures the energy loss of electrons as they pass through a sample.
It provides information about the elemental composition and chemical bonding of the sample.
EELS can be combined with electron microscopy to study the elemental composition of cellular structures.
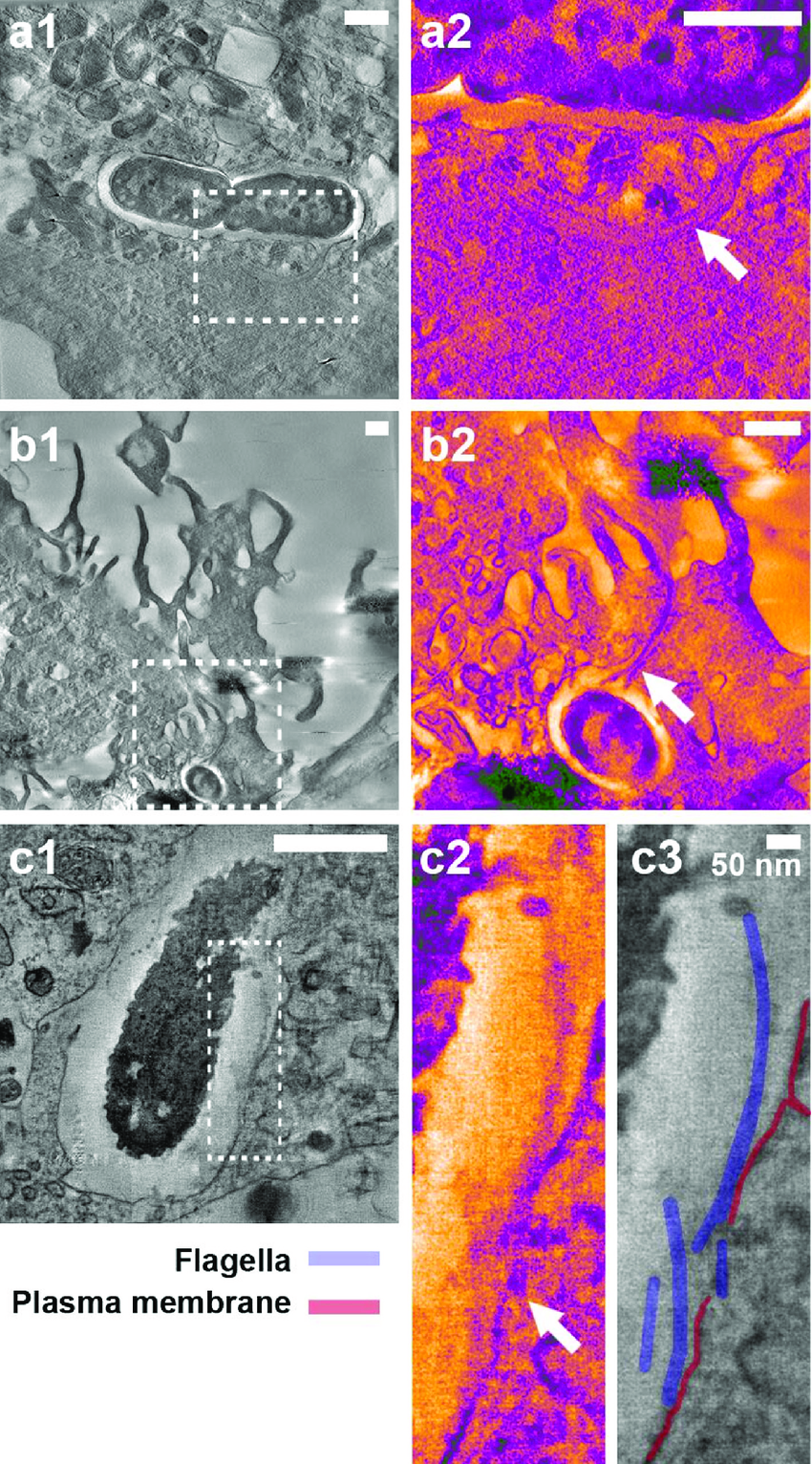
Electron Tomography:
Electron tomography is a technique used to create 3D reconstructions of cells and cellular structures.
It involves capturing a series of images from different tilt angles and then combining them to generate a 3D model.
Electron tomography allows for the visualization of complex cellular structures in their native context.
Single-Particle Analysis:
Single-particle analysis is a method used in cryo-EM to determine the 3D structures of macromolecules.
It involves imaging individual particles and aligning them to reconstruct a high-resolution 3D structure.
Single-particle analysis is particularly useful for studying large protein complexes and macromolecular assemblies.