Atoms, Ions, Isotopes
1/17
Earn XP
Description and Tags
Honors Physical Science
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
How do you find Mass Number?
By adding the number of protons and neutrons in a nucleus.
whats an atomic number made up of?
number of protons in a nucleus.
what is an electron cloud?
Electrons are most likely to be found in the area outside of an atoms nucleus, called an electron cloud. Electrons are an energy levels in the electron cloud.
how many electrons are in the 1st energy level?
2 electrons
How many electrons are in the 2nd energy level?
8 electrons
How many electrons are in the 3rd energy level?
8 electrons.
Do protons and neutrons have the same mass?
yes, meaning one AMU is also equal to the mass of one nuetron.
Octet rule
atoms want 8 valence electrons
What is an isotope?
An isotope is an atom of an element with the same number of protons but a different number of neutrons.
what is an ion?
an atom or molecule with a net charge, positive-cation, negative-anion.
what parts of an atom have mass?
protons and neutrons (not electrons)
What do atoms “want”
To have a full electron energy levels. Atoms with full electron energy levels are more stable and do not often react.

What is the atomic mass?
12.011
what is atomic mass?
The mass of an atom is so small that it needs its own unit. Atomic Mass Unit (amu) is the scale scientists use to measure the mass of atoms. One amu is equal to one proton.
Examples:Iodine(127), Nitrogen(14), Hydrogen(1)
How many electrons are in the 4th energy level?
18 electrons (e-)
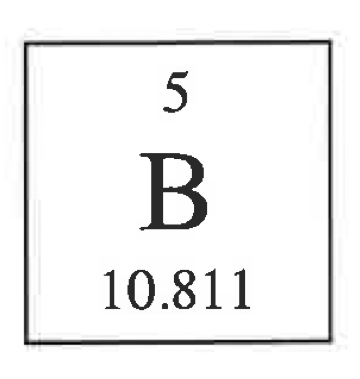
What’s the mass #, # protons, neutrons, and electrons?
11, 5, 6, 5