Electron Configuration for CHM 115 Exam 2
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
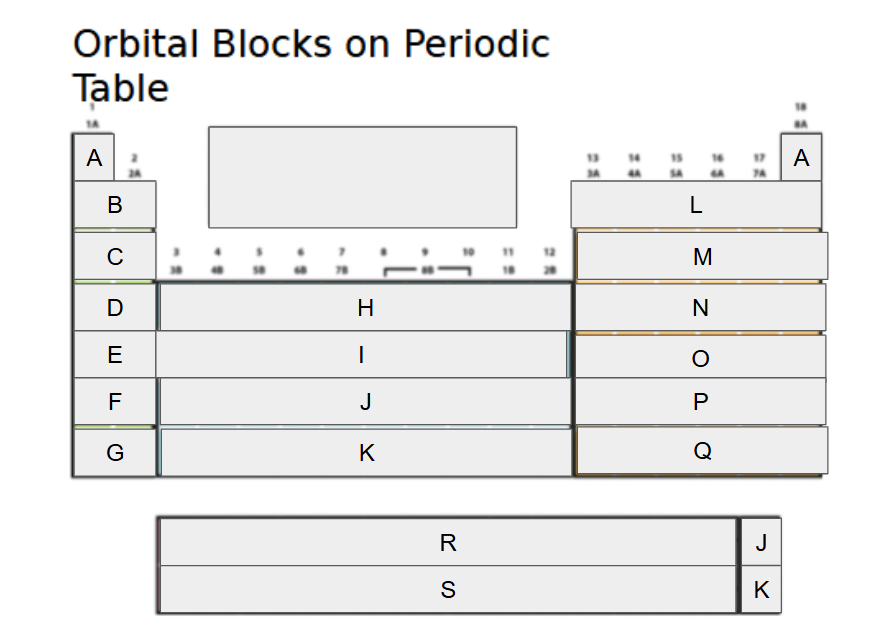
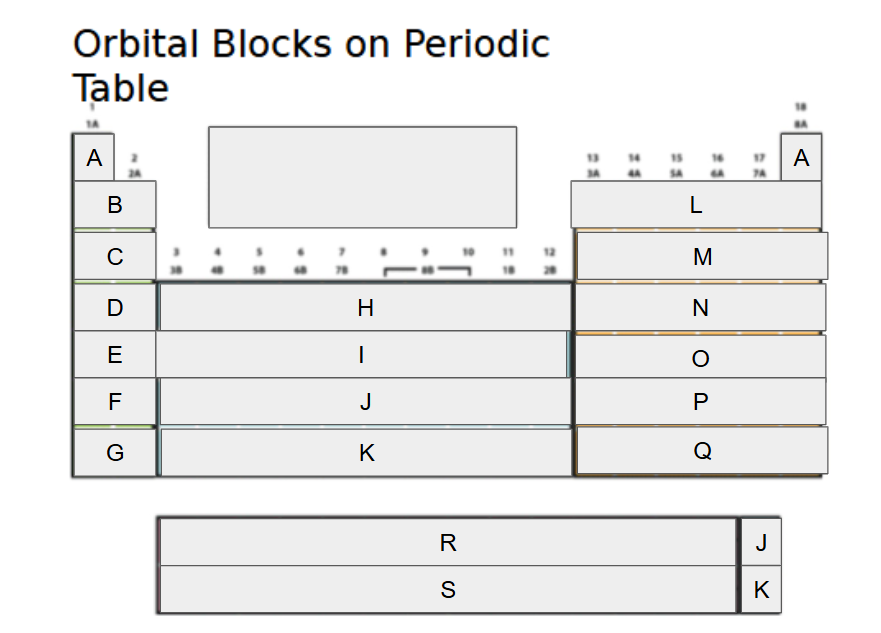
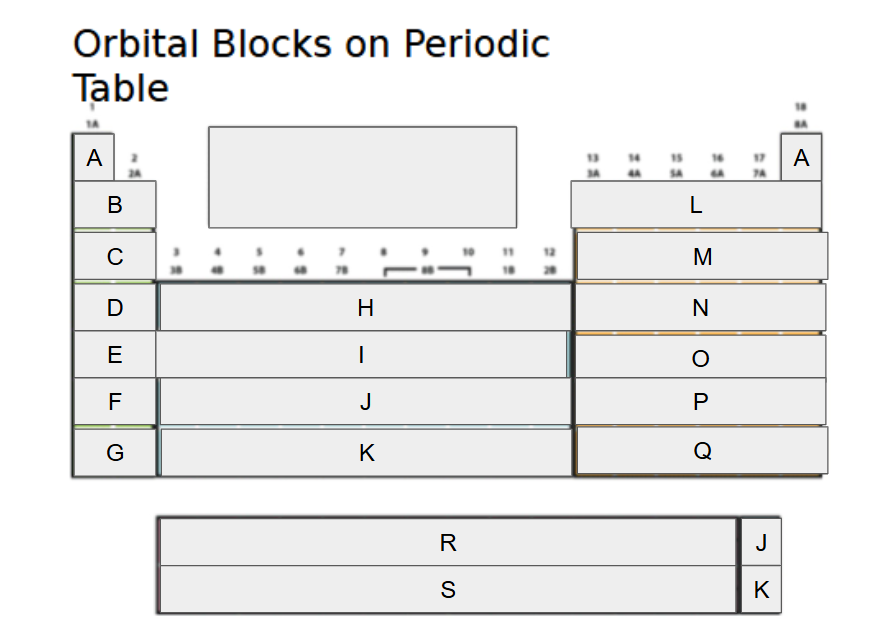
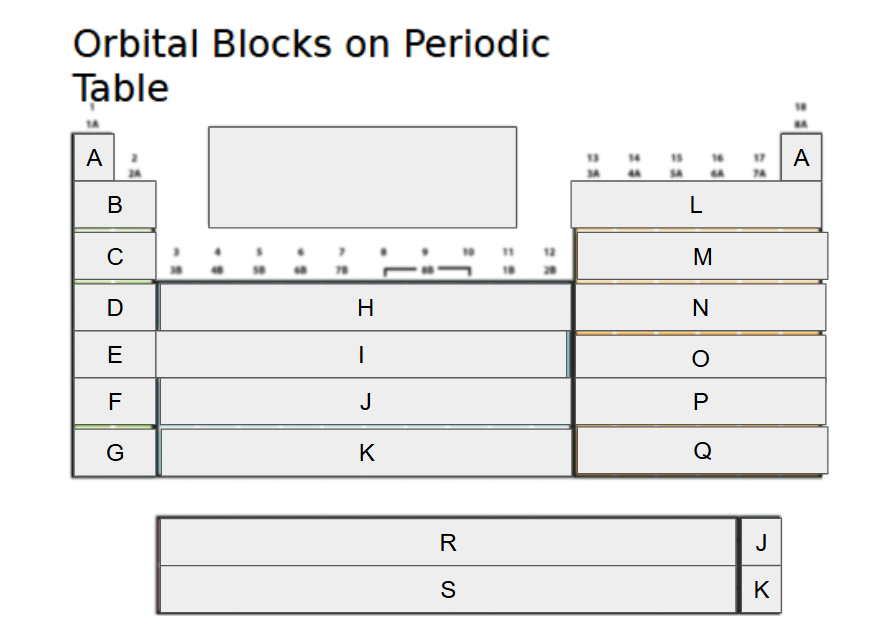
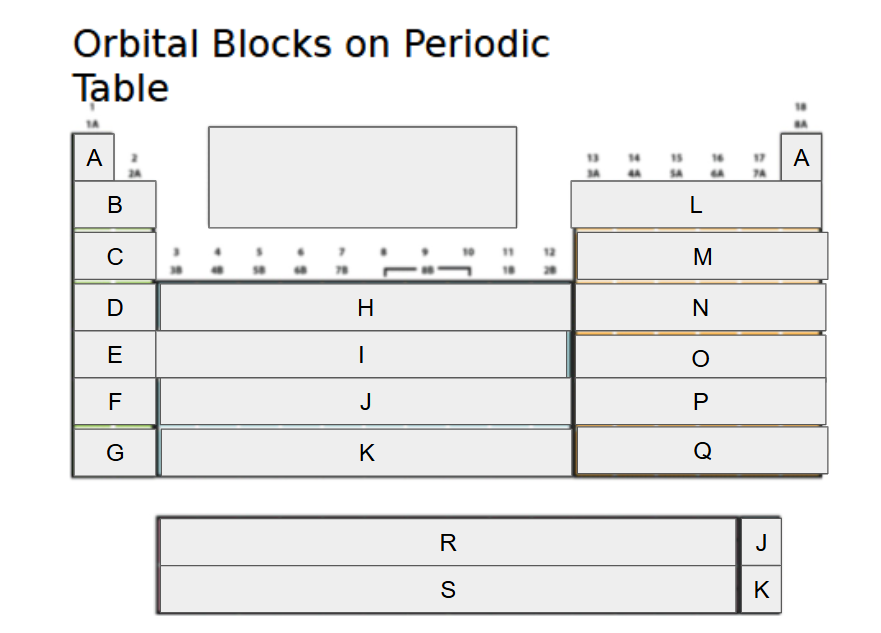
1s
What orbital block is A?
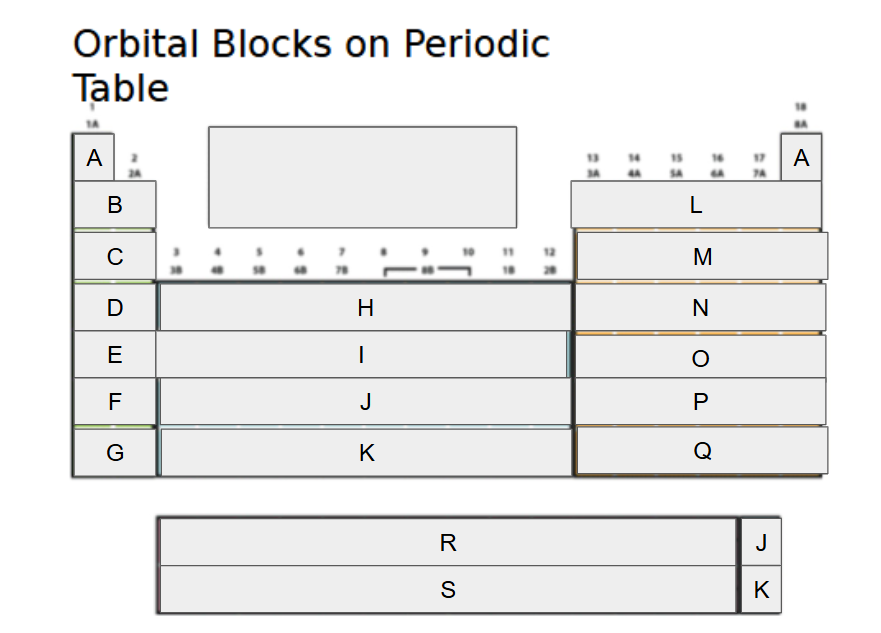
2s
What orbital block is B?
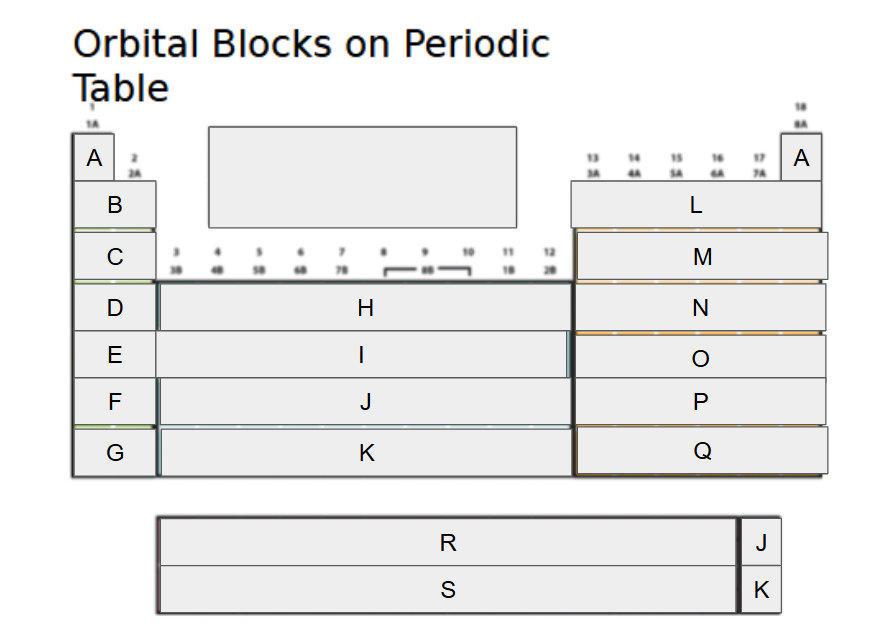
3s
What orbital block is C?
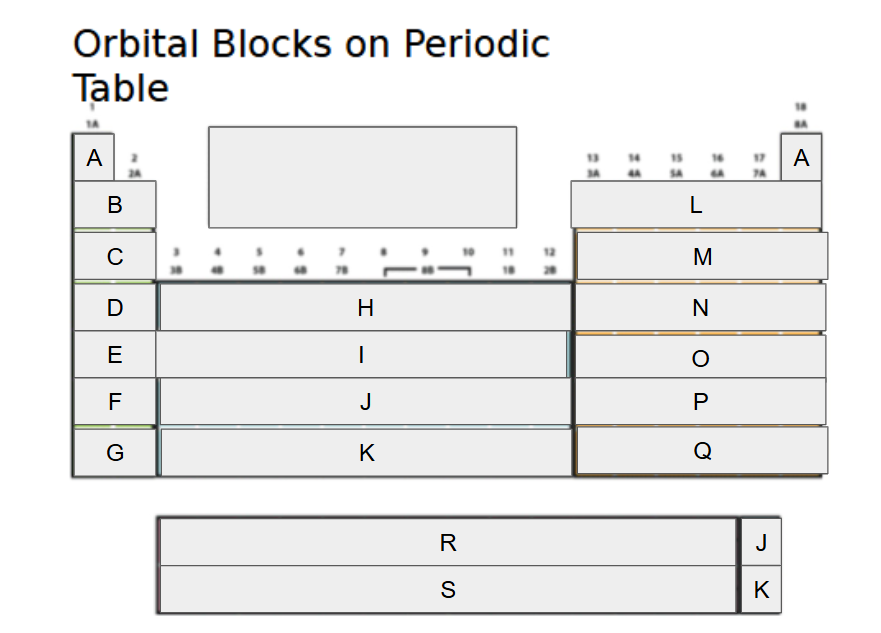
4s
What orbital block is D?
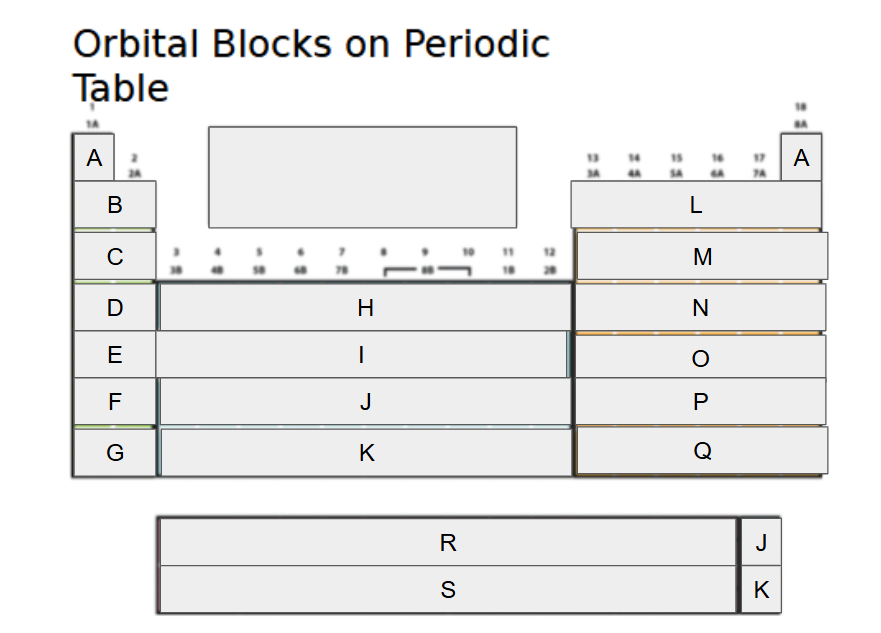
5s
What orbital block is E?
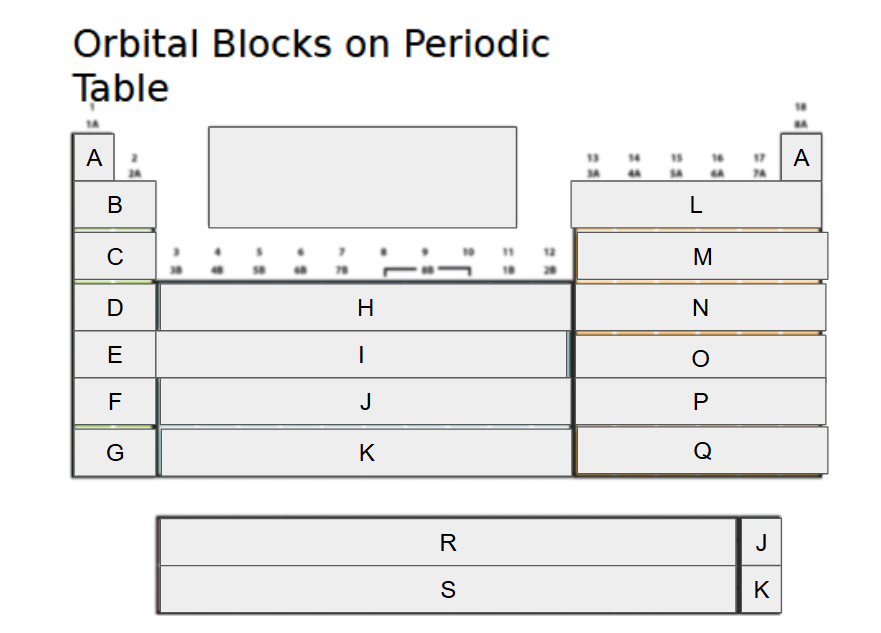
6s
What orbital block is F?
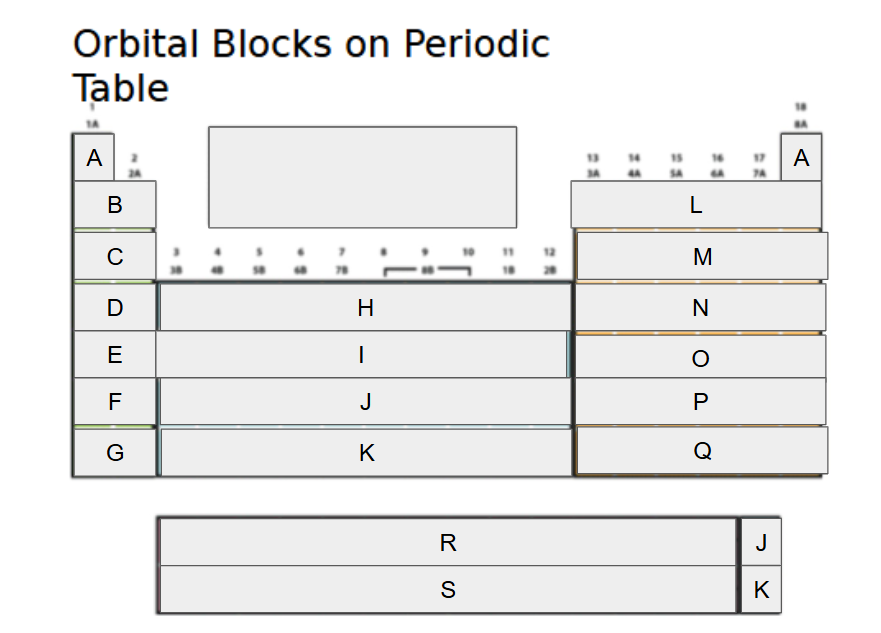
7s
What orbital block is G?

3d
What orbital block is H?
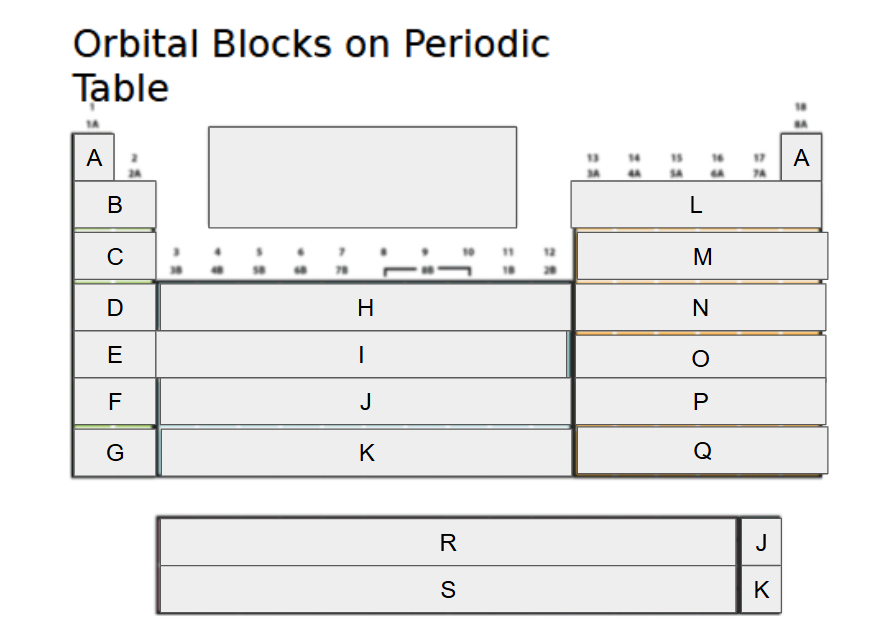
4d
What orbital block is I?
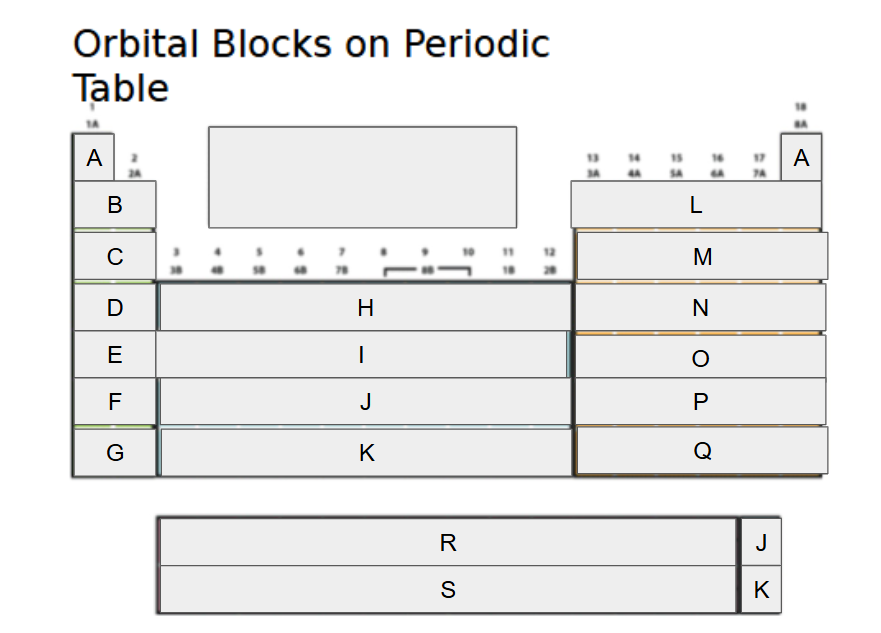
5d
What orbital block is J?
6d
What orbital block is K?
2p
What orbital block is L?
3p
What orbital block is M?
4p
What orbital block is N?
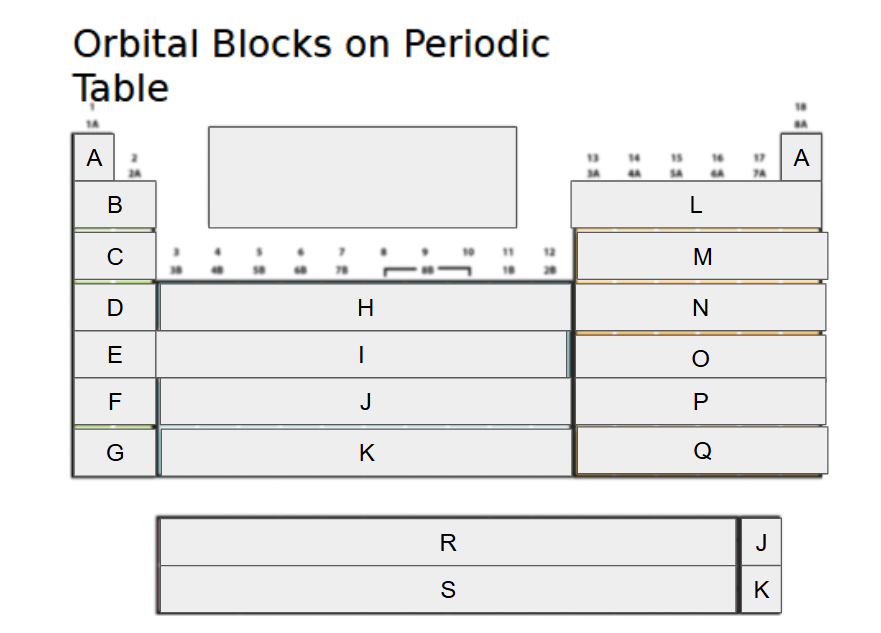
5p
What orbital block is O?
6p
What orbital block is P?
7p
What orbital block is Q?
4f
What orbital block is R?
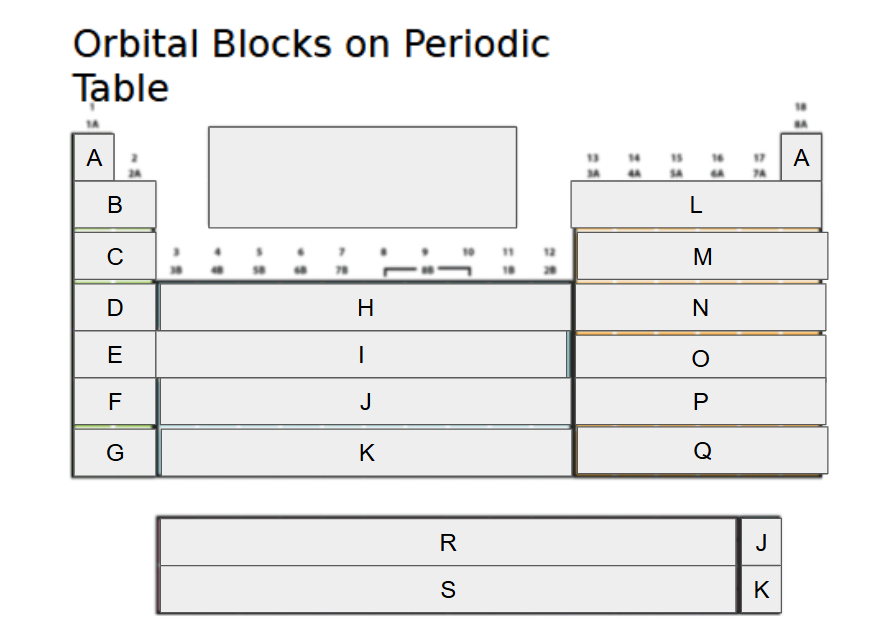
5f
What orbital block is S?
s-block
What Block is A-G?
d-block
What Block is H-K?
p-block
What Block is L-Q?
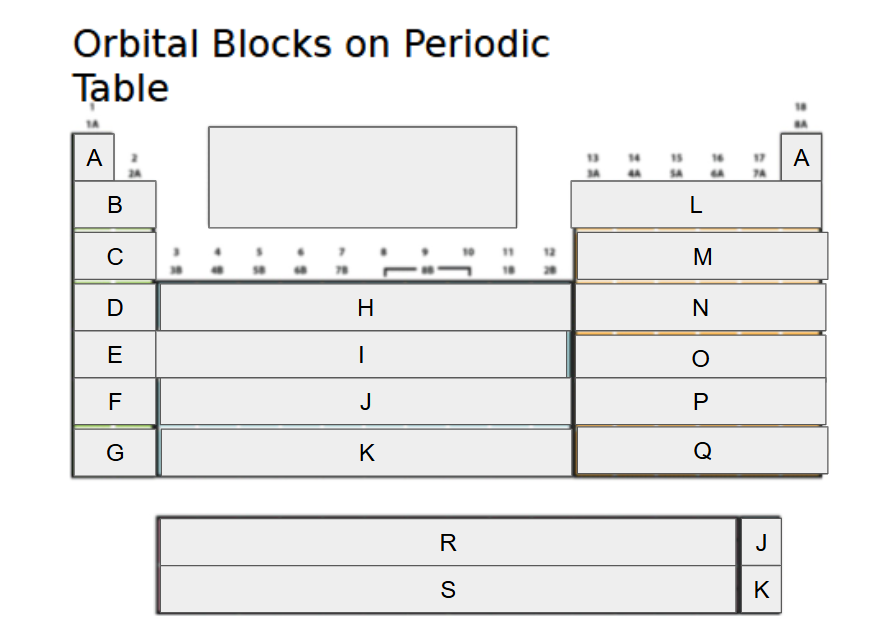
f-block
What Block is R-S?
1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s25f146d107p6
Write the order of the full electron configuration:
Isoelectronic
The species all have the same electronic configuration.
d and f sublevels
For Electron Configurations, completely filed __________ count as core
The Number of electrons in highest energy s and p sublevels
Only for main-group elements does the number of valence electrons equal:
Lowest Energy
Electrons are added to the atom in the order of the energy of the orbitals. The _____________ orbital fills first
highest energy orbital present
When a species has a positive charge, thus removing electrons, electrons are removed first from the:
True
True or False: s-orbitals are higher than d-orbitals