3.1.11 - Electrode potentials and electrochemical cells
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
59 Terms
RECAP:
What is a redox reaction?
One in which reduction and oxidation occurs in the same reaction
RECAP:
What is a disproportionation reaction?
reaction in which an element is both oxidised and reduced
RECAP:
What is an oxidising agent (oxidant)?
A substance that reduces itself to oxidise another species
RECAP:
What is a reducing agent (reductant)?
a substance that oxidises itself to reduce another species
When is an equilibrium set up in an electrochemical cell?
when a piece of metal (electrode) is dipped into a solution of its metal ions
What does it mean if the electrode has a negative potential?
the equilibrium lies to the left and the metal has a negative charge due to a build up of electrons on the metal
AANN: Oxidation/Anode/Negative
What does it mean if the electrode has a positive potential?
The equilibrium lies to the right and the metal has a positive charge as electrons have been used up to form metal from the metal ions
What does the position of the equilibrium depend on?
explain
Depends on the metal
reactive metals tend to form M2+ ions, so negative charge builds up on the metal = negative potentials
Unreactive metals tend to have positive charge on metal = positive potentials
Definition of a half-cell/electrode
A metal dipping into a solution of its ions
What metal electrode is used in a half-cell with no metal involved in the half-equation?
why?
platinum
inert
conducts electricity
If the surface area of the platinum electrode was doubled, what would happen to the emf of the cell?
it would remain unchanged
Definition of the electrochemical series
list of electrode potentials in numerical order
How do you measure the potential of an electrode?
combine two half-cells together (electrochemical cell) and find the potential difference between the two half-cells measured
What do you use to join two half cells together? why do you use these?
Voltmeter = to measure potential difference
Salt bridge = allows movement of ions to complete circuit
What is a salt bridge?
either:
a piece of filter paper soaked with a solution of unreactive ions
A tube containing unreactive ions in an agar gel
Why is a salt bridge needed?
It allows the movement of ions between electrodes to complete the circuit
What compounds are used for the salt bridge and why?
KNO3
Does not react with electrodes or electrolytes
What does SHE stand for? What is it?
SHE = Standard hydrogen electrode
An electrode assigned with 0 volts of potential and is used in comparison with other half-cells
What do electrochemical cells use?
uses electron transfer reactions i.e. redox to produce energy
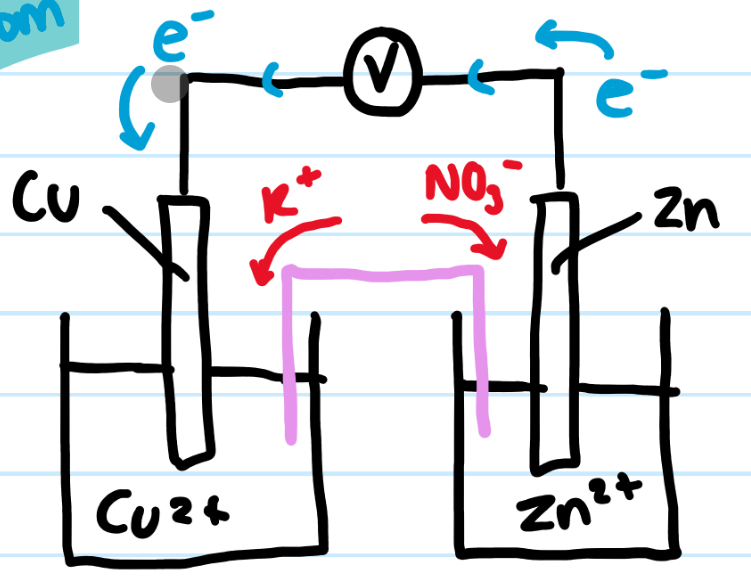
Draw the electrochemical cell of Cu(s)/Cu2+(aq) and Zn(s)/Zn2+(aq)
write the half equation of each half cell
Which direction do the electrons flow?
Name with half cell is oxidised and which is reduced
Name the anode and the cathode
What happens to the mass of each electrode
Give the overall equation
Cu2+(aq) + 2e- ⇌ Cu(s)
Zn2+(aq) + 2e- ⇌ Zn(s)
Electrons flow from Zn → Cu
Zn2+(aq) + 2e- ⇌ Zn(s) = OXIDATION
Cu2+(aq) + 2e- ⇌ Cu(s) = REDUCTION
ANODE = Zn (supply e-)
CATHODE = Cu (uses e-)
Zn decreases in mass
Cu forms on positive electrode = increases in mass
Zn + Cu2+ → Zn2+ + Cu
If given cell potential values how do you figure out which cell is oxidised and which is reduced?
NO PROBLEM
MORE NEGATIVE = oxidised
MORE POSITIVE = reduced
How do you calculate the total E°cell value?
Reduced E° cell value - Oxidised E° cell value
What happens to the total E°cell value/EMF if the concentration of ions in one electrode is increased?
why?
value: increases
more ions (be specific) to accept/donate e- (depends on either ions are reduced or oxidised)
Electrode becomes more positive/negative
What are the standard conditions of a half-cell?
cell concentration = 1 mol/dm3 of ions involved in half equation
Cell temperature = 298K
Cell pressure = 100kPa (only affects half-cells with gases)
Why is the potential exactly 0V under the standard conditions?
By definition
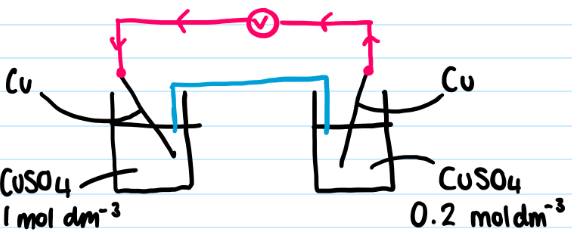
Explain what happens to the equilibrium of this cell that is not under stand conditions?
state which cell is oxidised and which is reduced giving the equation for both
more concentrated Cu2+ ions on left = eqm shifts to right
Left cell = reduction (+ve electrode) = Cu + 2e- → Cu2+
Less concentrated Cu2+ ions on right = eqm shifts to left
Right cell = oxidation (-ve electrode) = Cu2+ → Cu + 2e-
Order in which you write a conventional cell notation
Oxidation is on left in order of being oxidised
Reduction is on right in order of being reduced
|| means salt bridge
| separates phases (e.g. s, aq)
, between same phase
What is the exception to this order?
The SHE (standard hydrogen electrode) is always on the LHS
Write the cell notation for the following:
Cu/Cu2+ = +0.34V
Zn/Zn2+ = -0.76V
Zn(s) | Zn2+ (aq) || Cu2+ (aq) | Cu (s)
Write the cell notation for the following:
Fe2+/Fe3+ = +0.77V
H2/H+ = 0V
Pt (s) | H2 (g) | H+ (aq) || Fe3+ (aq), Fe2+ (aq) | Pt (s)
From the previous cell:
name the solutions used in each cell
H+/H2 = HCl
Fe2+/Fe3+ = FeCl2, FeCl3
When is a reaction feasible?
+ve value = feasible
-ve value = not feasible
From the electrochemical series explain the trend of:
Reducing agents
Oxidising agents
Best reducing agents = bottom (reducing ability increases down the series)
Best oxidising agents = top (oxidising ability decreases down the series)
Name 3 types of commercial cells
non-rechargeable
Rechargeable
Fuel cells
Briefly explain what a non-rechargeable cell is
Cell where the chemicals are used up over time and the emf drops
When one reactant is completely used up, the cell is flat and the emf is 0 volts
Cannot be recharged and have to be disposed after a single use
Name the two types of non-rechargeable cells you need to know
zinc-carbon
Alkaline
Zinc-carbon cell:
Characteristics of this cell
Give the two half equations involved in the cell
State which is oxidised/reduced
give overall equation
Cell emf
cheap but short life
Zn(NH3)22+ + 2e- ⇌ Zn + 2NH3
2MnO2 + 2H+ + 2e- ⇌ Mn2O3 + H2O
Zinc equation is oxidised
Second equation is reduced (more positive value in series)
2MnO2 + 2H+ + Zn + 2NH3 → Zn(NH3)22+ + Mn2O3 + H2O
0.70 - (-0.80) = +1.50V
Alkaline cell:
Characteristics of this cell
Give the two half equations involved in the cell
State which is oxidised/reduced
give overall equation
Cell emf
Higher cost cell, longer life
Zn2+ + 2e- ⇌ Zn
MnO2 + H2O + e- ⇌ MnO(OH) + OH-
Zinc equation is oxidised
Second equation is reduced (more positive value in electrochemical series)
Zn + 2MnO2 + 2H2O → 2MnO(OH) + 2OH- + Zn2+
0.84 - (-0.76) = +1.60V
Name the three types of rechargeable cells you need to know
lithium ion
Lead-acid
Nickel-cadmium
Lithium ion cell:
Uses
Give the two half equations involved in the cell
State which is oxidised/reduced
overall equation during discharge
Overall equation during re-charge
Cell emf
Cell notation
Phones, cameras
Li+ + CoO2 + e- ⇌ LiCoO2
Li+ + e- ⇌ Li
First equation is reduced
Second equation is oxidised
Li + CoO2 → LiCoO2 (normal overall equation (Li+ cancels out))
LiCoO2 → Li + CoO2 (reverse equation)
0.60 - (-3.00) = 3.60V
Li (s) | Li+ (aq) || CoO2 (aq) | LiCoO2 (s) | Pt (s)
LITHIUM ION CELL:
Write half equation for reaction at the negative electrode during operation
Li → Li+ +e-
LITHIUM ION CELL:
Suggest why water is not used as a solvent in this cells
Water would react with the lithium and create an explosion
LITHIUM ION CELL:
Suggest why the recharging of a lithium cell may lead to the release of carbon dioxide in the atmosphere
Electricity for recharging cell may come from power stations burning fossil fuels
Lead-acid cell:
Uses
Give the two half equations involved in the cell
State which is oxidised/reduced
overall equation during discharge
Overall equation during re-charge
Cell emf
In this reaction what does sulfuric acid act as?
Cars
PbO2 + 3H+ + HSO4- + 2e- ⇌ PbSO4 + 2H2O
PbSO4 + H+ + 2e- ⇌ Pb + HSO4-
First equation = reduced
Second equation = oxidised
2H+ + Pb + 2HSO4- + PbO2 → 2PbSO4 + 2H2O
2PbSO4 + 2H2O → 2H+ + Pb + 2HSO4- + PbO2
1.68 - (-0.36) = +2.04V
Electrolyte
Nickel-cadmium cell:
Give the two half equations involved in the cell
State which is oxidised/reduced
overall equation during discharge
Overall equation during re-charge
Cell emf
NiO(OH) + 2H2O + 2e- ⇌ Ni(OH)2 + 2OH-
Cd(OH)2 + 2e- ⇌ Cd + 2OH-
First equation = oxidation
Second equation = reduction
Cd + NiO(OH) + 2H2O → Ni(OH)2 + Cd(OH)2
Ni(OH)2 + Cd(OH)2 → 2H2O + NiO(OH) + Cd
0.52 - (-0.88) = +1.40V
What are fuel cells?
most common
continuous supply of chemicals into the cell so do not need recharging as never run out of chemicals
Most common fuel cell = hydrogen-oxygen fuel cell (which can be run in alkaline and acidic conditions)
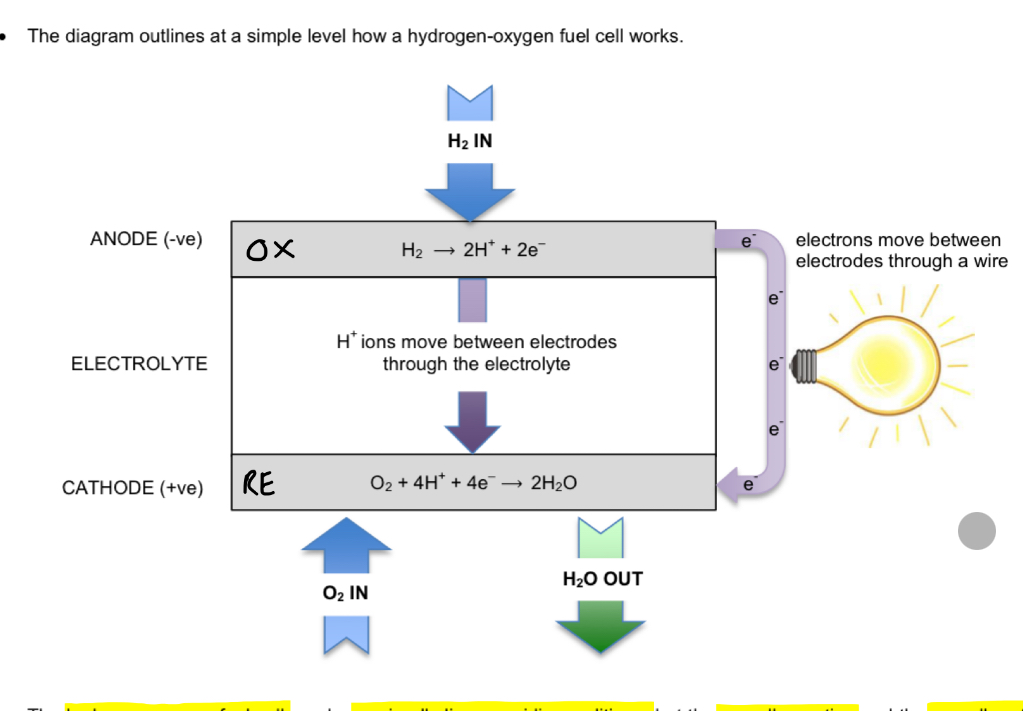
Hydrogen-oxygen fuel cell (alkaline conditions)
equation at negative electrode
Equation at positive electrode
Overall equation
Cell emf
Cell notation
H2 + 2OH- → 2H2O + 2e-
O2 + 2H2O + 4e- → 4OH-
2H2 + O2 → H2O
+1.23V
Pt (s) | H2 (g) | OH- (aq), H2O (l) || O2 (g) | H2O (l), OH- (aq) | Pt (s)
Hydrogen-oxygen fuel cell (acidic conditions)
equation at negative electrode
Equation at positive electrode
Overall equation
Cell emf
Cell notation
H2 → 2H+ + 2e-
O2 + 4H+ + 4e- → 2H2O
2H2 + O2 → H2O
+1.23V
Pt (s) | H2 (g) | H+ (aq) || O2 (g) | H+ (aq), H2O (l) | Pt (s)
Benefits and risks of:
using non-rechargeable cells
BENEFITS = cheap
RISKS = waste issues
Benefits and risks of:
using rechargeable cells
BENEFITS = Less waste, cheaper in long run, lower environmental impact
RISKS = some waste issues after useful life
Benefits and risks of:
using hydrogen fuel cells
BENEFITS = only waste product is water, do not need re-charging, very efficient
RISKS = need constant supply of fuels, hydrogen is flammable and explosive, hydrogen usually made using fossil fuels, high cost of fuel cells
Why do hydrogen fuel cell not need recharging
because fuel supplied continuously to cell so voltage output does not change
Explain why the current in the circuit of this cell falls to zero after the cell has operated for some time
(In a cell that has solutions with different concentrations)
Eventually the ions will be the same in each electrode
Suggest one reason why waste disposal centres have a separate section for cells and batteries?
pollution from toxic chemicals
If a question asks to give a reason (other than cost) why a cell is not recharged, what should you do?
See if it is reversible, if its not then it will not be rechargged
With a table of E°cell values how would you know which is the weakest oxidising/reducing agent?
Weakest oxidising agent = most negative value
Weakest reducing agent = most positive value
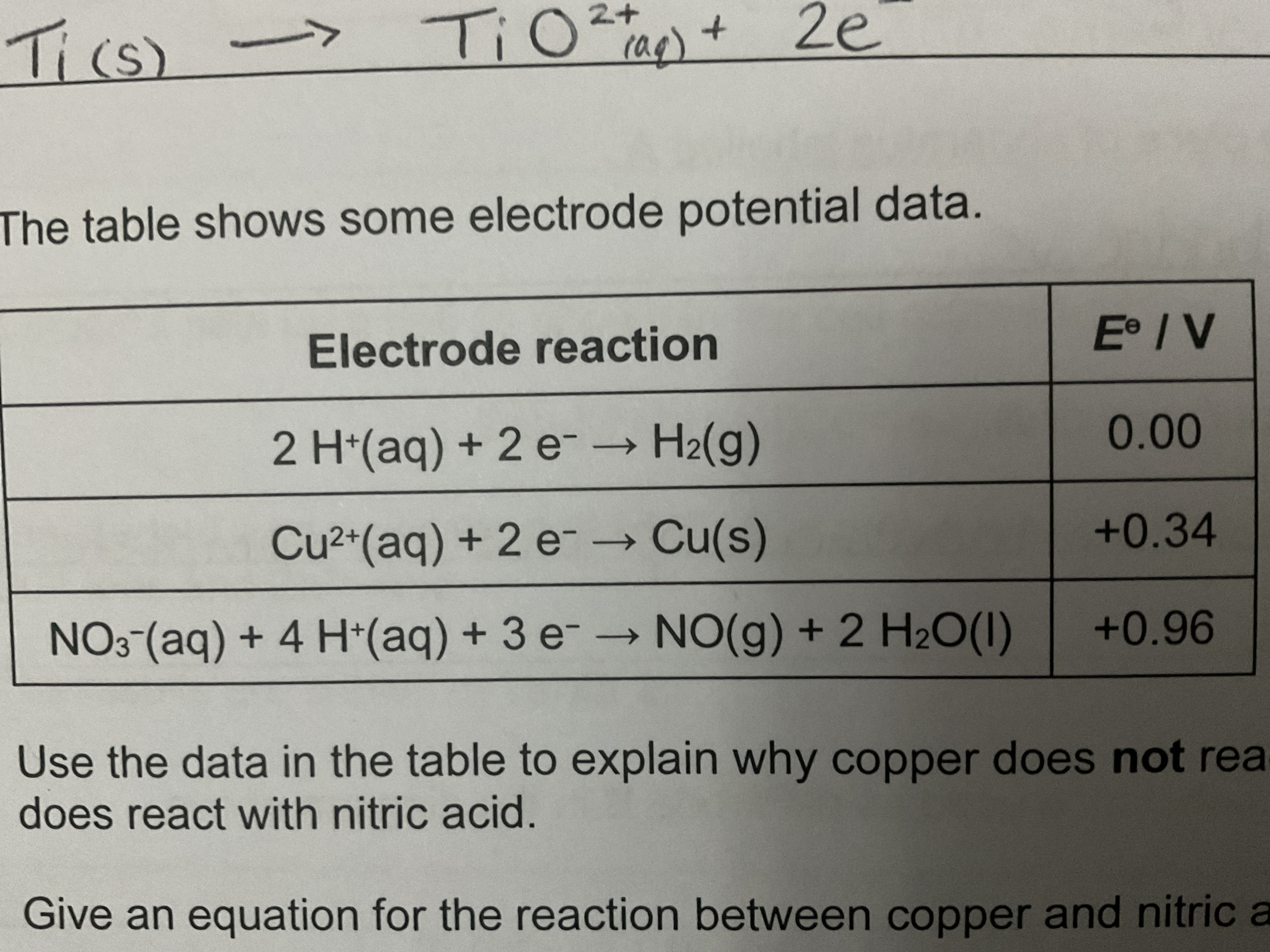
EXAM QUESTION: Use the data in the table to explain why copper does not react with most acids but does react with nitric acid
0.00 - 0.34 = -0.34V
E°cell for Cu with most acids (-0.34) is negative showing Cu is a less powerful reducing agent than H2
0.96 - 0.34 = +0.62V
E°cell for Cu with nitric acid is positive
State how you would change an electrochemical cell apparatus to allow the cell reaction to go to completion
remove voltmeter and add ammeter
Why would any electrode potentials that you calculate in experiments be different from the actual standard electrode potential for that electrode?
non standard conditions