Chemistry- Crude oil and Fractional Distillation
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What is crude oil made from? [2 marks]
Dead remains, and microscopic marine life.
How long does it take for crude oil to form? [1 mark]
Millions of years.
What process is used to separate crude oil? [1 mark]
Fractional distillation.
What is crude oil separated in? [1 mark]
A fractional column.
Draw a diagram of a fractional column. [3 marks]
A vertical tower, one pipe going in, several pipes coming out at different levels.
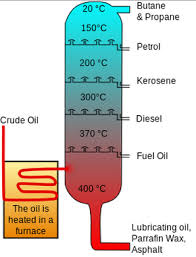
What happens to the crude oil as it enters the fractional column? [2 marks]
It is heated, and vaporised.
What is the temperature gradient of the fractional column? [2 marks]
Hotter at the bottom, cooler at the top.
What is a fraction? [2 marks]
A group of similar sized molecules, with similar boiling points.
What is the physical property of the different fractions that allows them to be separated? [1 mark]
They have different boiling points.
What is the relationship between the temperature of the fractional column and the boiling point of the fraction that condenses? [1 mark]
The hotter the temperature, the higher the boiling point OR the cooler the temperature, the lower the boiling point.
What is the relationship between the size of the molecules in the fractions and the boiling point? [1 mark]
The bigger the molecule, the higher the boiling point OR the smaller the molecule the lower the boiling point.
Why does the size of the molecule affect the boiling point? [1 mark]
Bigger molecules have stronger intermolecular forces OR smaller molecules have weaker intermolecular forces.