Chemical Reactions of Carboxylic Acids
1/10
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Acidity
Like alcohols, carboxylic acids evolve hydrogen gas when reacting with metal and form salts with alkalis. Unlike phenols, they require weaker base like sodium carbonate to evolve carbon dioxide
Smaller pKa value, stronger the acid
Trifluroacetic acid is the strongest organic acid (pKa = 0.23)
Carboxylic acids tend to be weaker than mineral acids but stronger than alcohols and phenols
Acidic nature of carboxyl group is due to the breaking of O-H bond, due to positively charged O weakening the bond as well as resonance stabilization of carboxylate ion
Effect of Substituents on Acidity of Carboxylic Acid
Electron withdrawing groups increase stability by dispersing negative charge via resonance effect
Electron donating groups decrease stability by intensifying negative charge
Directly substituted phenyl and vinyl actually increase stability despite decrease in resonance effect
Order of acidity for hybridization
sp3 > sp2 > sp
Order of increasing acidity
Ph < I < Br < Cl < F < CN < NO2 < CF3
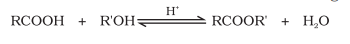
Esterification
Carboxylic acids react with alcohol/phenol in the presence of mineral acids like conc. H2SO4 and HCl as a gas (as catalyst) to produce esters
Mechanism
Protonation of carbonyl oxygen
Nucleophilic addition of alcohol
Loss of water molecule and proton
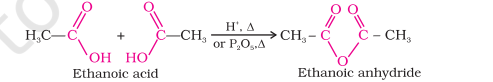
Anhydride Formation
Carboxylic acids heat with mineral acids like conc. H2SO4 or P2O5 to give us anhydrides
Acid anhydrides are formed by the elimination of a water molecule
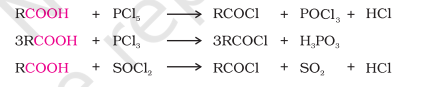
Reaction with Phosphorus Trihalides, Phosphorus Pentahalides and Thionyl Chloride
Like alcohols, hydroxyl group is replaced with Cl using reagents like PCl5, PCl3, SOCl2
SOCl2 is the preferred reagent because the other two products obtained are gaseous giving easy purification
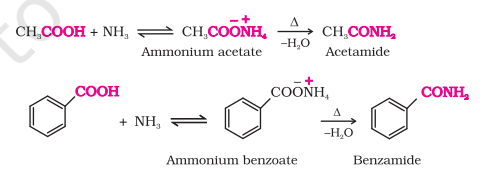
Reaction with Ammonia
Carboxylic acids react with ammonia to give ammonium salts that on further heating give amides
Reduction
Carboxylic acids can be reduced to primary alcohols using LiAlH4 or more easily diborane
Diborane has difficulty to reduce functional group like ester, halo, nitro
NaBH4 does not reduce carboxyl group
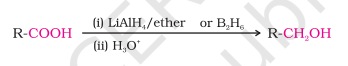
Decarboxylation
Using soda lime
Carboxylic acids form hydrocarbons when sodium salt is heated with soda lime (NaOH and CaO in ratio 3:1)

Decarboxylation
Kolbe’s Electrolysis
Aqueous solutions of alkali metal salts of carboxylic acids undergo electrolysis to form hydrocarbons having twice the number of carbon atoms compare to alkyl group of acid
Halogenation
Hell-Volhard-Zelinsky Reaction
Carboxylic acids with α-hydrogen react with chlorine and bromine substituted at α position in the presence of red phosphorus to give α-halocarboxylic acids

Ring Substitution
Aromatic carboxylic acids undergo electrophilic substitution where carboxyl group is deactivating and meta-directing
Do not undergo Friedel-Crafts because the carboxyl group is deactivating and the catalyst, aluminum chloride get attached to carboxyl group