Orgo Lab C: Separation of a ternary mixture using the Extraction technique
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
What is extraction?
A technique used for the isolation and purification of organic compounds.
What’s a typical solvent mixture?
Non-polar organic + polar aqueous
What’s the density of water?
1.0g/mL
What’s the density of tBMe?
0.74g/mL
Draw the compounds used in Lab C: benzoic acid, 2-napthol, 1,4-dimethoxybenzene
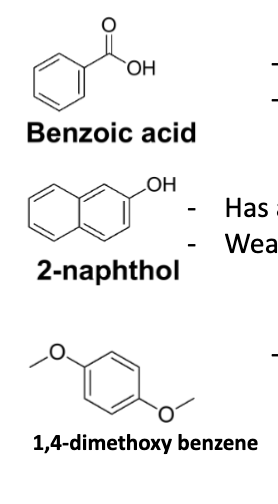
pKa of benzoic acid and 2-napthol?
Benzoic acid = 4.17 (strong acid)
2-napthol = 9.5 (weak base)
1,4-dimethoxybenzene = neutral
Draw the structure of caffeine
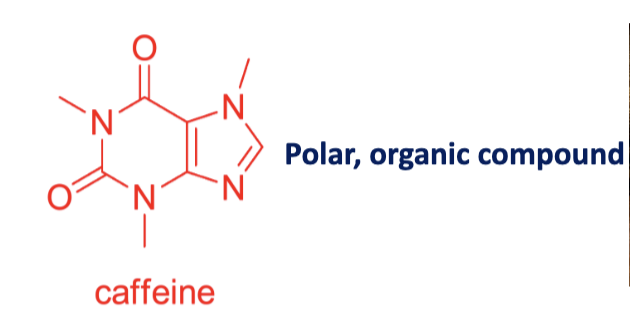
Solubility of caffeine?
2.2g/100mL at 25°C, polar organic compound
Solubility of dichloromethane? CH2Cl2
10.2g/100mL, polar organic solvent
What forces control solubility?
Van der Waals forces
Dipole-Dipole forces
Hydrogen bonding
Like dissolves like
Formula for partition coefficient K

% recovery formula

What’s the theory of extraction?
It is always better to do several smaller extractions than one big extraction.
- To maximize the amount of material extracted, and in turn to increase the
theoretical recovery.
Draw the diagram for separatory funnel (open + closed stopcock)
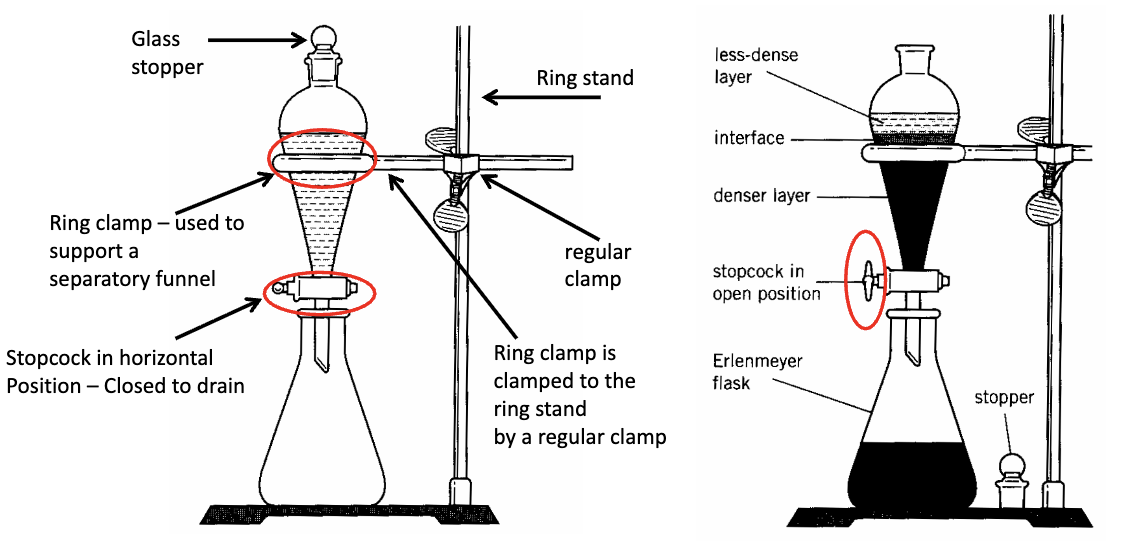
What do you do before draining?
Remove glass stopper
What size separatory funnel + glass stopper do we use for lab C?
125 mL separatory funnel w/ 19/22 standard taper joint; 19/22 glass stopper.
19 = width, 22 = length in mm
What kind of funnel do you use when adding solutions to a separatory funnel?
Pyrex funnel (long)
Density of water and dichloromehane?
Water, d=1.00g/mL
Dichloromethane, d=1.33g/mL
What are drying agents?
Anhydrous salts (devoid of water) combining w/ water that is mixed with your product + retains it as water of crystallization.
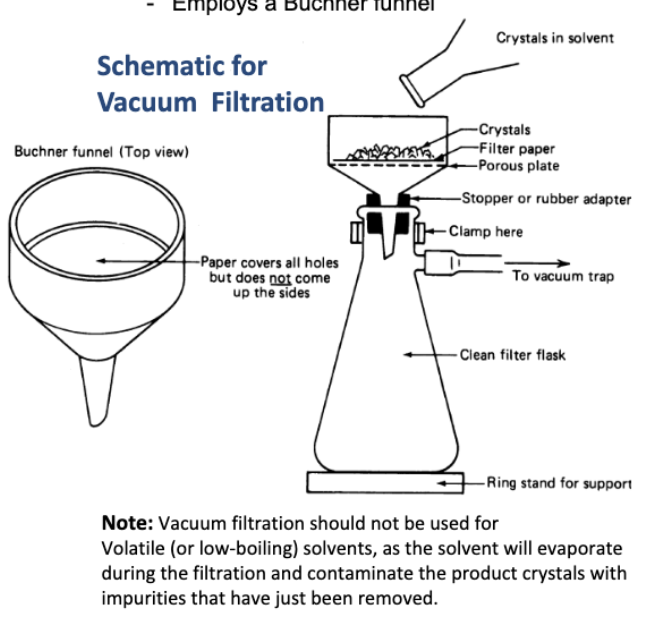
When do you choose gravity filtration over vacuum filtration?
When you use a low-boiling solvent (vacuum can evaporate solvent).
Draw the gravity filtration setup
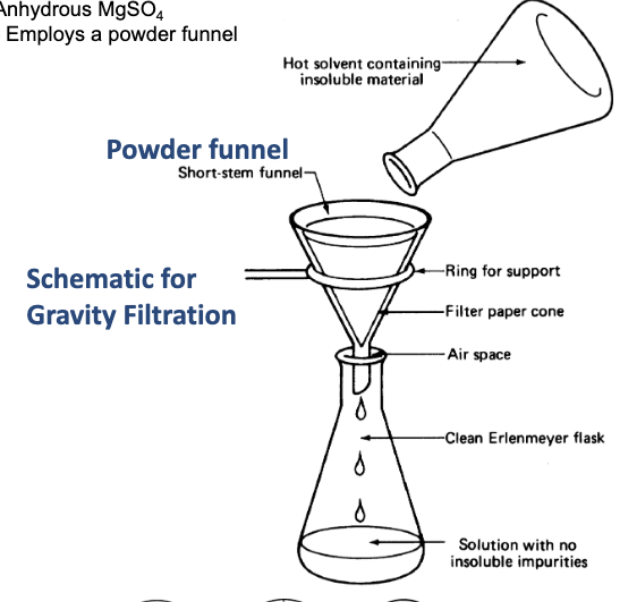
Draw the vacuum filtration setup
Draw the rotary evaporator setup
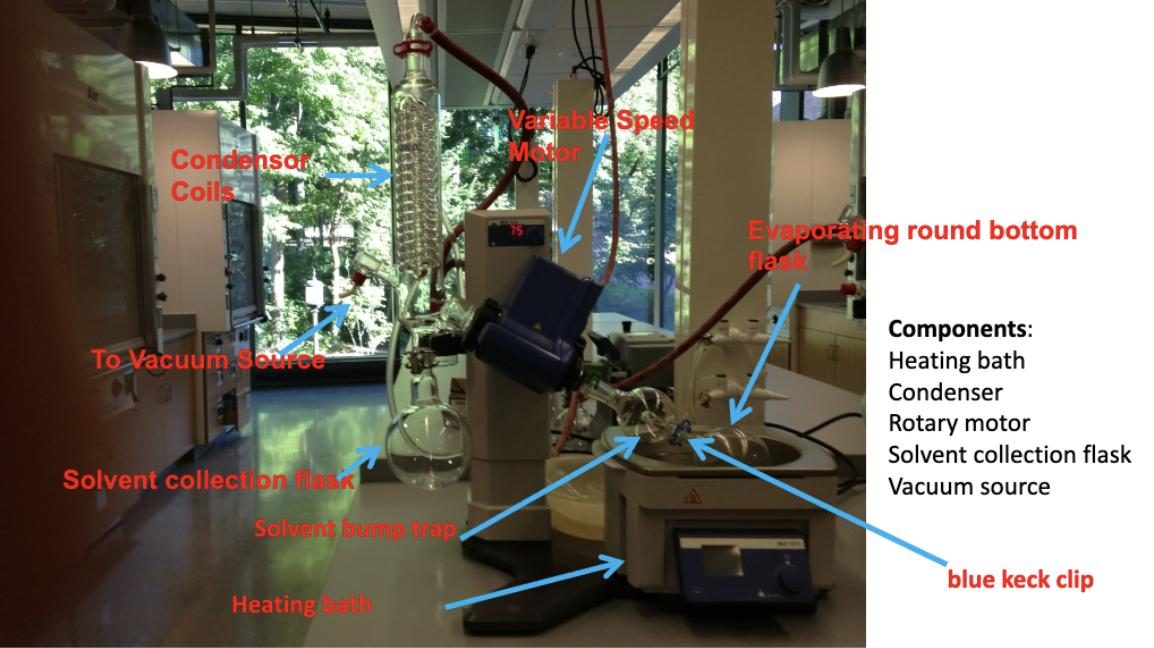
List the rotary evaporator precautionary measures:
Do not use a round bottom flask w/ any visible cracks.
RB flask should not be more than half-full (solvent/product could spill into solvent bump trap).
Don’t use broken blue keck clip (can slip into hot water bath).
What are emulsions?
A suspension of immiscible droplets of one liquid in another liquid. Results in a loss of two distinct phases.
How do emulsions form?
Shaking the separatory funnel too vigorously. But shaking too gently may not give you an effective mixing of the two phases.
How do you get rid of an emulsion?
Let the separatory funnel sit undisturbed in ring clamp for a few mins, stir emulsion interface w/ glass rod, add a little salt.
How does a rotary evaporator work?
Spins RB flaks to increase SA covered by solvent. Applies vacuum to reduce BP of solvent being removed. Spinning flask helps prevent solvent from bumping.