Chemistry - Central Science C1
1/72
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
73 Terms
Ag
Silver
Sb
Antimony
Sn
Tin
Pb
Lead
He
Helium
Pt
Platinum
Co
Cobalt
Br
Bromine
V
Vanadium
Hg
Mercury
Matter
Something that has mass and takes up space
Heterogeneous Mixture
Not uniform throughout
Homogeneous Mixture
Parametric composition (variable) and uniform throughout
Pure Substance
A sample of matter that has fixed composition (is not parametric, is uniquely categorized by its properties)
Compound Substance
A pure substance formed by more than one kind of atom
Element
A pure substance made of only one kind of atom
Physical properties
A characteristic of a pure substance that can be observed without changing it into another substance
Chemical properties
Characteristic that cannot be observed without altering the substance
Intensive properties
Does not depend on the amount of matter present (e.g. boiling temperature)
Extensive properties
Depend of the amount of matter present (e.g. mass and volume)
Physical Change
A change in a substance that does not involve a change in its composition
Chemical Changes (or Chemical Reaction)
A substance is transformed into a chemically different substance
Volatile
Lower boiling point relative to other substance
Distillation
A process that separates liquid mixtures based on their differing boiling points. Involves heating the mixture to vaporize the more volatile component, then cooling the vapor to condense it back into liquid form, thereby isolating the components of the mixture.
Chromatography
A technique that is used to separate the components of a mixture based on the tendency of each component to travel or be drawn across the surface of another material.
Density of Diamond
3.5 g/cm3, greater than water, smaller than zirconia
Diatomic Elements
H2, N2, O2, F2, Cl2, I2, Br2 (Have No Fear Of Ice Cold Beer)
Energy
The ability to do work
Potential Energy
Energy that is stored and held in readiness
Chemical Energy
A form of potential energy that is stored in chemical bonds between atoms
Electrostatic Energy
Potential energy that results from the interaction of charged particles
Breaking Chemical Bonds
Consumes energy (increasing potential energy)
Creating Chemical Bonds
Releases energy
Unit of Mass
kilogram (kg)
Liter
10e-3 m³
If no temperature reported, for density and volume, it should be assumed
25⁰C
Joule (J)
SI unit of energy (kg-m²/s²; [E_K] = [1/2mv²] = [m][v²]=kg-m²/s²)
calorie (cal)
4.184J. Historically known to be the amount of energy needed to raise temperature 1 gram of Water from 14.5 to 15.5 ⁰C, also known as 15-degree calorie or cal_{15}
Calorie (Cal)
Equal to 1kcal
Kelvin to Celcius
K = C + 273.15
10e18
exa (E)
10e15
peta (P)
10e12
tera (T)
10e9
giga (G)
10e6
mega (M)
10e-6
micro (μ)
10e-9
nano (n)
10e-12
pico (p)
10e-15
femto (f)
10e-18
atto (a)
Precision
The degree to which repeated measurements are consistent with each other.
Accuracy
The degree to which a measurement is close to the true value.
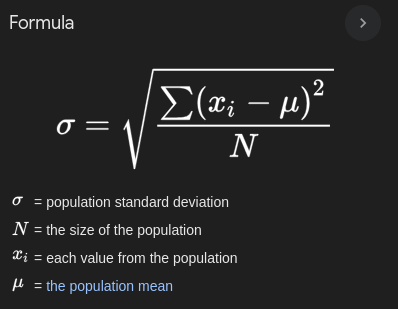
Standard Deviation
A statistical measure that quantifies the amount of variation or dispersion in a set of data values. It indicates how much individual data points differ from the mean.
68-95-99.7 rule (or empirical rule)
A statistical rule stating that for a normal distribution, approximately 68% of data points fall within one standard deviation, 95% within two, and 99.7% within three standard deviations from the mean.
Difference between 5000 and 5000.
The former has more significant digits than the latter. Indicating good accuracy.
Law of Constant Composition
States that the elemental composition of a pure compound is always the same.
Work
The energy transferred to or from an object via the application of force along a displacement
Work (formula)
W = F * D
Kinetic Energy (formula)
KE = 1/2 m v²
Gravitational Energy (formula)
GE = m g h
Solution
A homogeneous mixture of two or more substances, where one substance (the solute) is dissolved in another (the solvent)
Filtration
A physical separation process that removes solid particles from a liquid or gas using a filter medium that allows the fluid to pass through while retaining the solids.
Rhenium
Re
Tungsten
W
Caesium
Cs
Indium
In
Arsenic
As
Xenon
Xe
Krypton
Kr
Tellurium
Te
Germanium
Ge
Mohs Scale
A scale used to rank the hardness of minerals, ranging from 1 (talc) to 10 (diamond)
Zinc
Zn