Chapter 27: Environmental Microbiology
The Hydrological (water) Cycle - The cyclic exchange of water between the atmosphere and biosphere.
Water precipitates as rain which then falls to earth → returns to the air by evaporating
As water flows through and on the ground, it carries nutrients both beneficial and harmful
Carbon runoff accelerates microbial respiration which causes O2 becomes depleted
Example of Heterotrophy
A metric used to evaluate or gauge metabolic activity would be Biochemical oxygen demand (BOD). Microbial oxygen consumption creates a biochemical oxygen demand (BOD).
Biochemical oxygen demand (BOD): amount of O2 removed from the environment by aerobic respiration.
You can see how active these microbes are being breaking down material through BOD.
When measuring O2 with dissolved oxygen probe, the rate of decrease is proportional to the organic matter concentration.
If there is more organic matter concentration, there would be more oxygen consumption and therefore a higher BOD.
If there is less, there would be less organic oxygen consumption and therefore lower BOD.
High BOD levels can threaten viability of fish & other aquatic animals when O2 levels are < 5 mg/L
Air saturated water = 8 mg/L O2 → that’s as much O2 as is can hold
Eutrophication results in high levels of aerobic, bacterial decomposition of organic matter.
Occurs from an influx of excess nutrients that trigger hypoxic zones in water
Think of runoff water bringing fertilizer into a body of water which brings about a boom in growth. Once the nutrients brought from the runoff is depleted, the growth dies off and falls to the bottom. Heterotrophic bacteria then feeds off the dead mass. The aerobic respiration from the bacteria breaking down the dead mass depletes oxygen from the water which causes fish and other life to die.
The purpose of wastewater treatment plant is to produce a clear outgoing stream, possessing minimal levels of organic solutes
Wastewater treatment - decreases the BOD and the level of human pathogens before water is returned to local rivers.
Water undergoes:
Preliminary treatment - removes solid debris
Primary treatment - fine screens & sedimentation tanks remove insoluble particles. EX: sediments
Secondary treatment - microbial decomposition of organic content.
Tertiary (advanced) treatment - chlorination or other chemical application to eliminate pathogens
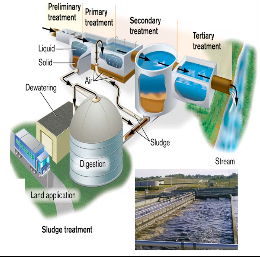
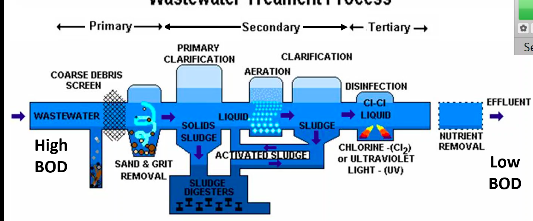
Water Treatment Process
About enhancing the growth of heterotrophic, aerobic respirers
Clarification - knock down high BOD along with removing the organic matter causing the high BOD (settle out) → those organic matter is recycled into the activated sludge which is then used to help lower high BOD
Activated Sludge - a process for treating wastewater using air and a biological floc composed of bacteria and protozoa
Healthy sludge - a brown floc, largely composed of saprotrophic bacteria (feed on decayed matter) but also an important protozoan flora
composed of amoebae, ciliated protozoan, stalked ciliates, and others
floc formation is critical in promoting settling of bacteria & associated microbes from treatment process.
yields abundant, clarified effluent
Filamentous bacteria important in floc formation; optimal ratio of filamentous to planktonic types
The microbes in flocculation will eat free floating microbes
We want microbes to settle so flocculation is what we want and is a good thing
The conversion of nitrate (NO3-) to N2 is called denitrification
Ammonia/ammonium ion (NH3/NH4+) would be the end products of nitrogen fixation and ammonification
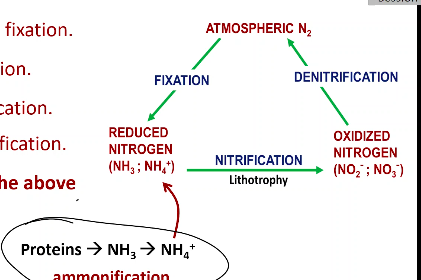
Nitrogen has more oxidation states than any other major biological elements
Some forms of nitrogen is as a food source or a way to breath
More reduced forms of nitrogen or those with more electrons are better used for food sources
More oxidized is better for respiration
The nitrogen cycles also cannot exist without prokaryotes
Lithotrophy/nitrification (assimilation) → food sources
ammonia is turned into nitrates
Denitrification (dissimilation) → ways to respire.
Results in N2 which is released to the air.
Nitrogen fixation - brings back the nitrogen lost from the denitrification and turns it into Ammonia
Ammonification - decomposing plants and animals turn into ammonia which is then inserted into nitrification
The major accessible nitrogen source is the atmosphere, of which N2 constitutes 79%
Artificial nitrogen fixation is accomplished by the Haber process. → generates fertilizers for agriculture: N2 + 3H2 → 2NH3
How you fix in ammonia artificially
Uses high pressure and catalysts. Very energy consuming
The nitrogen cycle can be viewed as a nitrogen triangle
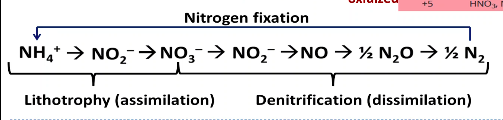
In fixation, the symbiotic types do the most of the activity of bringing in the nitrogen in the air. → After the nitrogen from the air is converted into reduced nitrogen (one of which is ammonia) → Nitrification turns them into oxidized nitrogen (NO2-;NO3-) → Denitrification uses oxidized nitrogen which turns them into atmospheric nitrogen
Nitrogen Fixation
N2 → NH3 → NH4+
NH4+ is assimilated into amino acids
Nitrogen fixation is catalyzed by nitrogenase
Only functions anaerobically
requires tremendous input of energy N2 is three bonded and takes lots of energy to break those bonds down
Occurs in all ecosystems & widespread across many bacterial species → free living-types and symbiotic types (ones who have relationships with plants)
Symbiotic - bacterial associations with certain plant types
Cyanobacteria in oceans and freshwater; compartmentalizes N2 fixation in heterocysts - strictly nitrogen fixation.
heterocysts are separated from other cells because of the photosynthetic cells. since oxygen would cause nitrogenase to become inactive and photosynthesis produces oxygen, they have to be kept seperated.
Nitrification
NH4+ → NO2- → NO3-
Lithotrophy - oxidation of NH4+ provides electrons, energy
In soil, Nitrosomonas oxides NH4+ to NO2-
Nitrobacter oxidizes NO2- to NO3-
excessive fertilizer use causes nitrate runoff; eutrophication of streams, danger to water supplies
Can also change soil pH or water pH
Denitrification
NO3- → NO2- → NO → N2O → N2
represents anaerobic respiration
leads to loss of nitrogen
reducing nitrogen