What is Organic Chemistry?
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Organic Chemistry
Study of molecules that have carbon atoms
Catenates
Bonds to itself
Organic
Means “derived from living things”
Jons Jacob Berzelius
Coined the term “organic chemistry”
Organic Chemistry (Back then)
Referred to the study of chemical compounds extracted from living things.
We thought that organic compounds could only be harvested from living things.
Friedrich Wohler
Discovered that an inorganic salt, ammonium cyanate, could be used to make urea without a living organism.
Organic Chemistry (Today)
The study of the structure, properties, composition, reactions, and preparation of carbon-containing compounds.
This definition includes chemicals extracted from living things, but also man made polymers, like plastics.
Organic chemistry is _________
carbon-centric
Lewis Structure
Illustrates what atoms are connected.
Show all of the bonds and lone pairs of electrons in a molecule.
Molecular Formula
Tells us the atoms we have and how many of each atom there is.
Doesn’t tell us what’s bonded to what.
Condensed Structural Formula
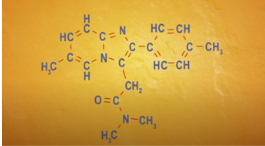
Skeletal Formula
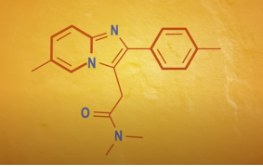
Skeletal Formulas
Carbons are the bends or the ends of the lines
Hydrogens aren’t shown because carbon atoms in most organic compounds have 4 bonds, so the number of hydrogen needed to give each carbon 4 bonds is implied
Makes it easier for us to focus on the parts of an organic structure that are non-carbon atoms or have double or triple bonds
Heteroatoms
Any atom that is not carbon or hydrogen