Balancing Chemical Equations.
0.0(0)
Card Sorting
1/3
Earn XP
Description and Tags
https://www.youtube.com/watch?v=vxCyzR6uETs&ab_channel=Freesciencelessons
Last updated 11:19 AM on 4/12/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
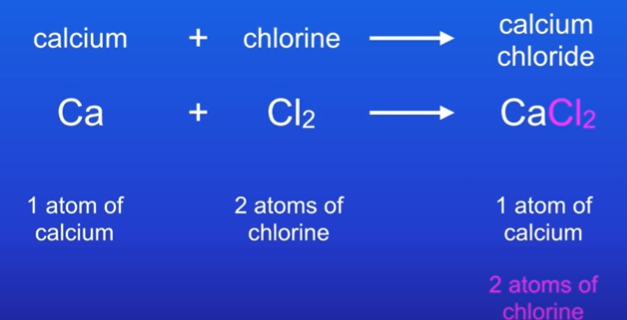
What does a balanced equation look like.
It has the same amount of atoms on both sides.
2
New cards
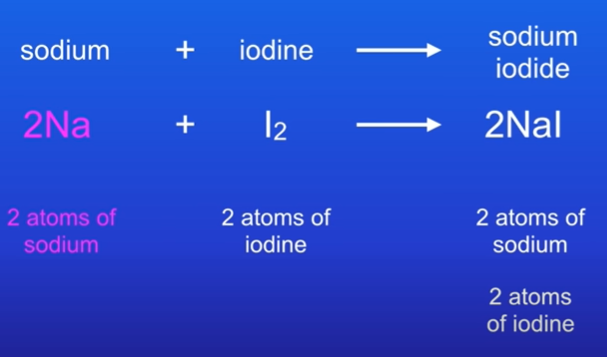
How do you balance the equation sodium + iodine → sodium iodide.
Na + I2 → NaI
On left hand, we have 1 atom of sodium and on right we also have 1 sodium atom.
On left hand, we have 2 atoms of iodine, but only 1 on the right.
We can place a 2 on the right hand side to get 2 atoms of iodine, which also gives us 2 atoms of sodium
We can fix this by adding another 2 infront of the sodium on the left side.
3
New cards
Method for balancing
Check each atom at a time, and write down how much are on left and right, then balance after.
4
New cards
a
a