Chapter 21: Buffers and Neutralisation
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
25 Terms
what is a buffer solution?
a system that minimises the pH change when small amounts of acid or base or added
2 ways of preparing a weak acid buffer solution:
weak acid + its salt
partial neutralisation of the weak acid
acid buffer solution prep 1 → weak acid + salt
mix a weak acid, like ethanoic acid, with a solution of one of its salts
when ethanoic acid is added to water, the acid partially dissociate
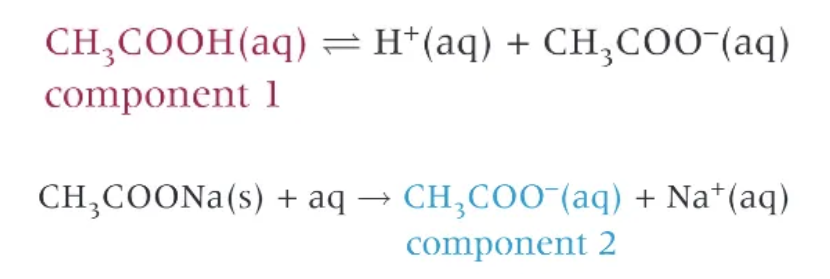
acid buffer solution prep 2 → partial neutralisation of the weak acid:
add aqueous solution of an alkali to the excess of weak acid.
weak acid is partially neutralised by the alkali, forming the conjugate base
some of the weak acid is left over unreacted → resulting solution contains a mixture of the salt of the weak acid and any unreacted weak acid
conjugate base removes an acid:
On the addition of H+ (aq):
[H+(aq)] increases.
H+(aq) ions react with conjugate base, A- (aq)
the equilibrium position shifts to the left, removing most of the H+(aq) ions.
![<p>On the addition of H+ (aq):</p><ol><li><p>[H+(aq)] increases.</p></li><li><p>H+(aq) ions react with conjugate base, A- (aq)</p></li><li><p>the equilibrium position shifts to the left, removing most of the H+(aq) ions.</p></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/83b8d811-b883-4646-ab61-7108d5f4e134.png)
weak acid removes an added alkali:
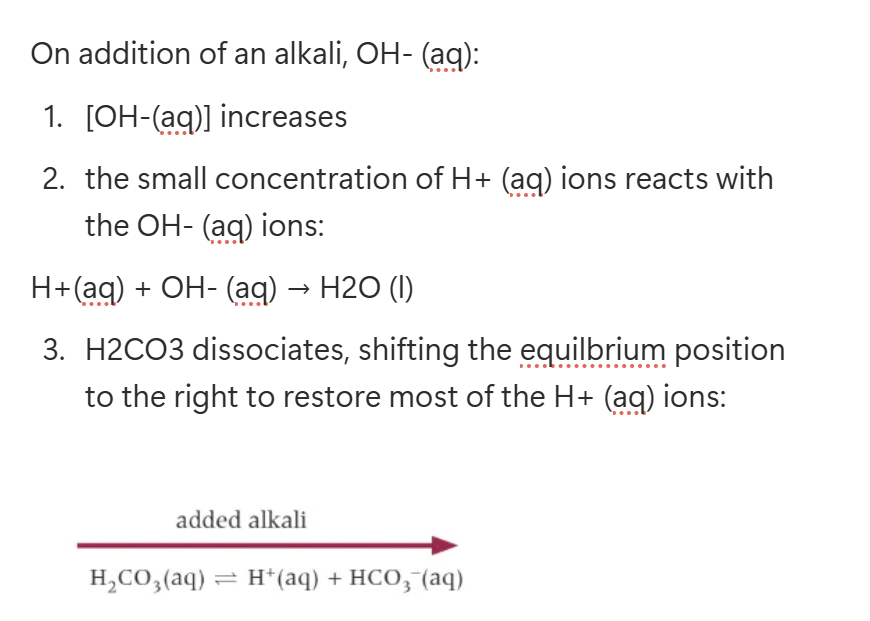
On the addition of alkali, OH- (aq):
[OH-(aq)] increases.
The small concentration of H+ (aq) ions reacts with the OH-(aq) ions:
H+(aq) + OH-(aq) → H2O (l)
HA dissociates, shifting the equilibrium position to the right to restore most of the H+ (aq) ions.
![<p>On the addition of alkali, OH- (aq):</p><ol><li><p>[OH-(aq)] increases.</p></li><li><p>The small concentration of H+ (aq) ions reacts with the OH-(aq) ions:</p></li></ol><p>H+(aq) + OH-(aq) → H2O (l)</p><ol><li><p>HA dissociates, shifting the equilibrium position to the right to restore most of the H+ (aq) ions.</p></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/b75858f5-a8fa-44df-8253-df05abb2e1c1.png)
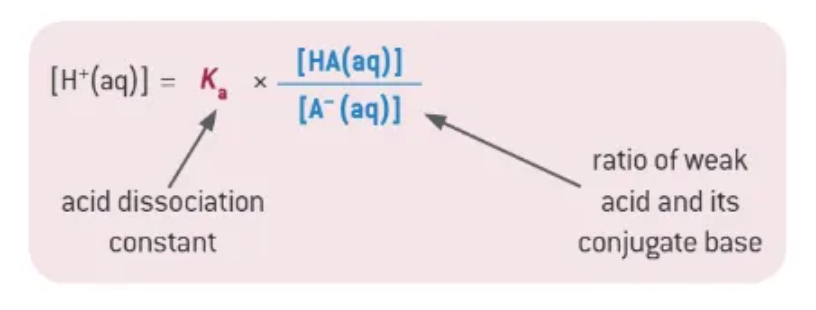
a buffer is most effective at removing either added acid or alkali…
when there are equal concentrations of the weak acid and its conjugate base
When [HA] = [A-]:
the pH of the buffer solution is the same as the pKa value of HA
the operating pH is typically over about two pH units, centred at the pH of the pKa value.
what can be assume for a weak acid, but not for a buffer?
the approximation that [H+] = [A-]
buffer Ka equation:
normal, healthy blood should have a pH of…
7.40
if blood pH falls below 7.35, people can get….
if blood pH rises above 7.45, people can get:
acidosis
alkalosis
acid being added to the blood:
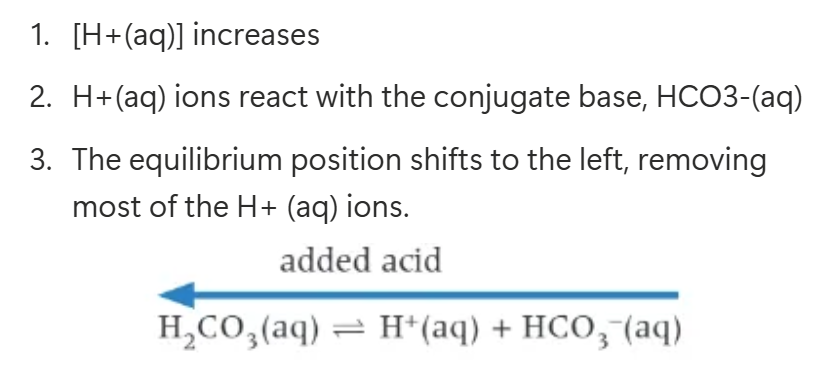
alkali being added to the blood:
the body prevents H2CO3 build up by…
converting it to carbon dioxide gas, which is then exhaled by the lungs
how to use a pH meter:
using a pipette, add a measured volume of acid to a conical flask
place the electrode of the pH meter in the flask
add the aqueous base to the burette and add to the acid in the conical flask, 1cm3 at a time.
after each addition, swirl the contents. Record the pH and the total volume of the aqueous base added.
repeat steps 3 and 4 until the pH starts to change more rapidly. then add the aqueous base dropwise for each reading until the pH changes less rapidly.
now add the aqueous base 1cm3 at a time again until an excess has been added and the pH has been basic, with little change, for several additions.
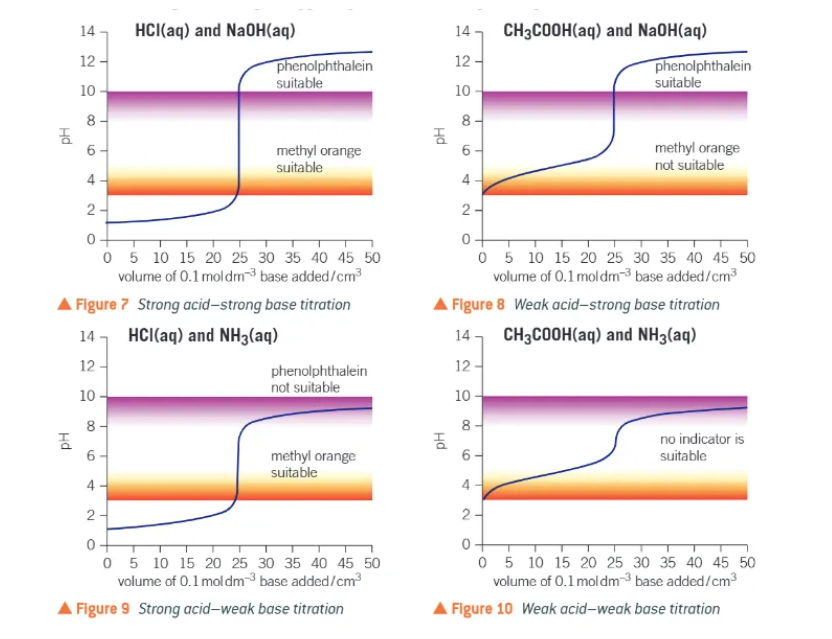
the pH titration curve:
when the base is first added, the acid is in great excess.
pH increases verrrrry slightly
as the vertical section is approached, the pH starts to increase more quickly as the acid is used up more quickly
pH increases rapidly → the acid and base concentrations are similar
after the vertical section, the pH will rise very slightly as the base is now in great excess.
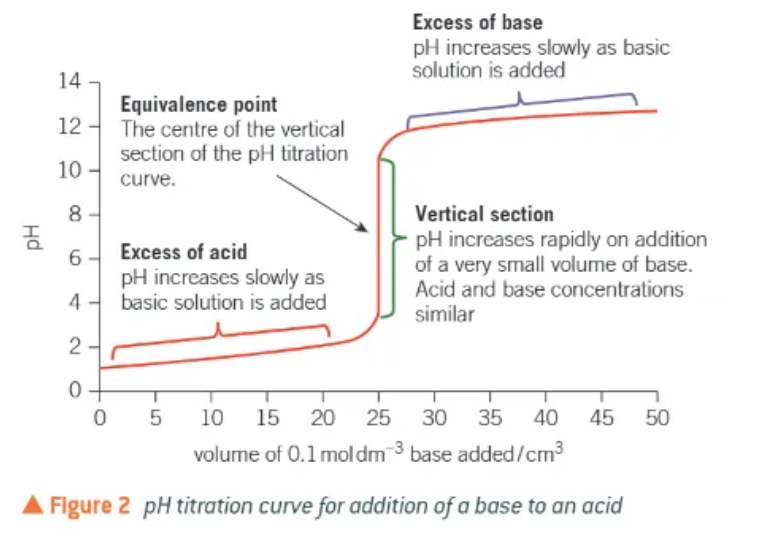
what is the equivalence point?
the volume of one solution that exactly reacts with the volume of the other solution
matches the stoichiometry of the equation
the centre of the vertical section of the pH titration curve
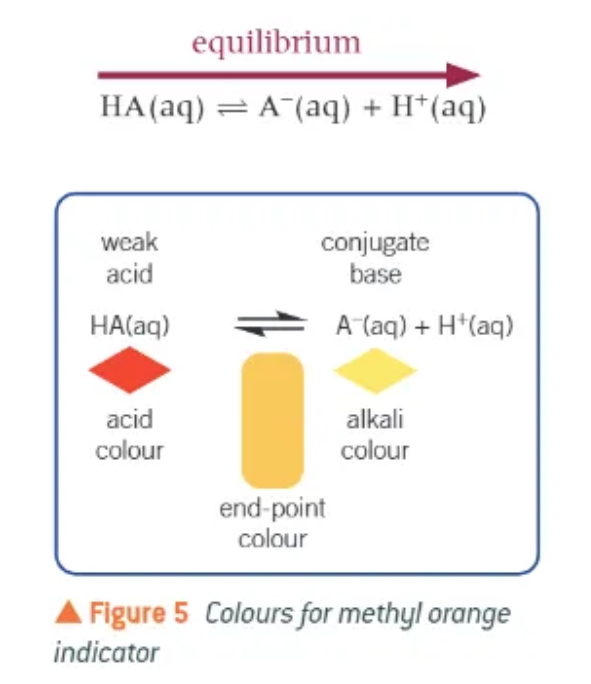
methyl orange colours:
red in acid
yellow in alkali
explain indicator colour changes:
indicator = weak acid
equilibrium shifts towards the weak acid in acidic conditions or towards conjugate base in basic conditions → changes colour as it does so
in a titration adding a strong acid to a strong base, methyl orange is initially…
red → the presence of H+ ions forces the equilibrium positions well to the left
methyl orange changing colour when alkali is added:
OH-(aq) ions react with H+(aq) in the indicator.
H+(aq) + OH-(aq) → H2O (l)
the weak acid, HA, dissociates + shifts the equilibrium to the right
the colour changes, first to orange at the endpoint and finally to yellow as the equilibrium positions if shifted to the right.
methyl orange changing colour when acid is added:
H+(aq) ions react with the conjugate base, A-(aq)
the equilibrium position shifts to the left
the colour changes, first to orange at the endpoint and finally to red when the equilibrium position has shifted to the left

how to choose an indicator?
the colour change must coincide with the vertical section of the pH titration curve. Ideally, the endpoint and the equivalence point would coincide.
what do pH curves look like?