Metal alloys for fixed appliances
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
46 Terms
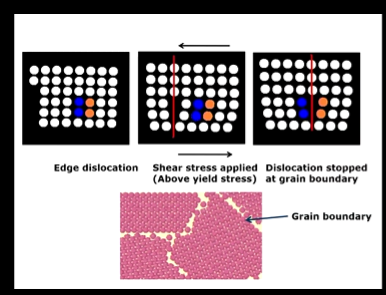
What are dislocations?
Imperfections within metals, all metals will have these imperfections
e.g a missing plane of atoms - line defect
applying a shear stress above the yield stress - permanently deforming the metal through movement of a dislocation from one plane to another within the crystal until it reaches a grain boundary where it cannot move anymore - so dislocations stack up at the grain boundary -
while deforming a metal you are increasing the density of dislocations - which makes it harder to deform the metal as you have to move extra deformations
Give examples of strengthening mechanisms? (7)
Baseline: Increasing the dislocations within the metal, making it harder as well as decreasing the ductility
Work (strain) hardening (Cold working): application of shear stresses produces more dislocations, hinders movement, more difficult to deform
Forging: Drawing wires/rolling sheets
Bending: Denture clasps/orthodontic appliances
Burnishing: Amalgam/Gold inlays
Heating treatments:
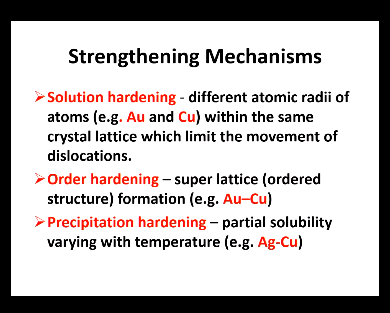
Solution hardening - Different atomic radii of atoms (e.g Au and Cu) within the same crystal lattice limit the movement of dislocations (dislocations cannot move as you are creating obstacles)
Order hardening: Super lattice - heating gives energy and time for atoms to rearrange each other to give an ordered structure formation (only one phase)
Precipitation hardening - partial solubility varying with temperature, as temp goes down another phase will precipitate so dislocation will not move beyond this (2 phases)
Requirements of metals and alloys? (6)
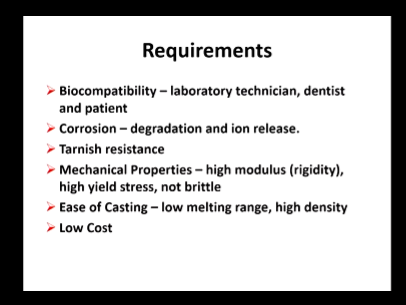
Biocompatible - lab tech, dentist and pt
Corrosion - degradation and ion release
Tarnish resistant - change in colour
Mechanical properties - high modulus and yield stress, not brittle
East of casting - low Mp and high density (easier to flow and remove any air)
Low cost
modulus means
Classification of metals used?
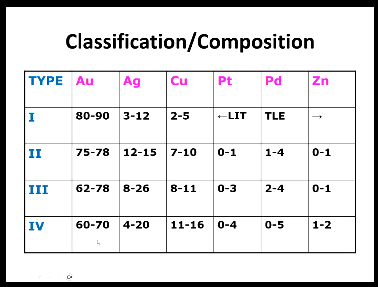
High noble: more than or equal to 60% noble metal, of which 40 or more needs to be gold
[Au, Pt, Pd, Rh, Ru, Os, Ir] (silver is not considered a metal alloy because it corrodes and tarnishes in the oral environment)
Noble - must contain at least 25% noble metal
Predominantly base metal - contain < 25% noble metal
![<p><strong>High noble:</strong> more than or equal to 60% noble metal, of which 40 or more needs to be gold</p><p>[Au, Pt, Pd, Rh, Ru, Os, Ir] (silver is not considered a metal alloy because it corrodes and tarnishes in the oral environment)</p><p><strong>Noble</strong> - must contain at least 25% noble metal</p><p><strong>Predominantly base metal</strong> - contain < 25% noble metal </p>](https://knowt-user-attachments.s3.amazonaws.com/5521bb9b-71c9-4e7a-b9dd-954743e253b1.png)
What are alloys used in Fixed pros?
Gold alloys:
- High gold - type 1,2,3 and 4 (discussed in removable pros)
- Medium and low gold
Palladium alloys:
- Silver-palladium
- High palladium (Pd-Cu)
Nickel (cobalt) - chrome alloys
Titanium and its alloys

High gold alloys
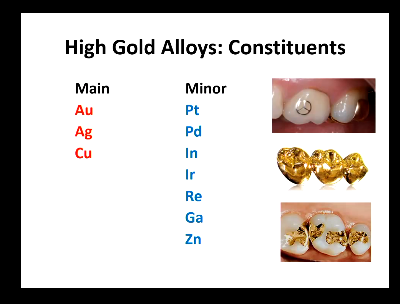
what are the main (3) and minor (7) constituents?
Main: Au, Ag, Cu
Minor: Pt, Pd, Ir, Zn, In, Ga, Re
Why is Ag added, what can it do?(4)?
Solution hardening
Precipitation hardening
Whitens
Reduces tarnish resistance
What does Cu add? (3)
Lowers Mp
Solution hardening
Order hardening (providing more than 11% Cu)
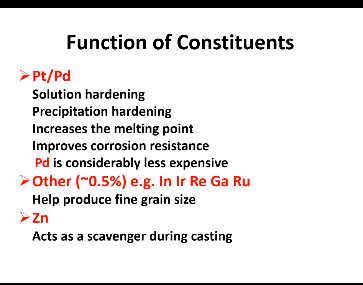
Pt/Pd functions? (4)
which is cheaper?
Solution hardening
Solution precipitation hardening
Increases Mp
Improve corrosion resistance
Pd is considerably less expensive
Function of Zn?
Acts as a scavenger during casting
In, Ir, Re, Ga Ru? function? (1)
Help produce fine grain size
High gold types composition?
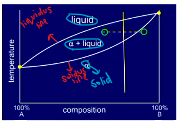
All the additions of metal form what with Au? (gold)
What does Pt and Pd addition cause in the graph which requires what?
All additions form solid solutions with Au
Pt and Pd increase the solidus/liquidus gap so type 3 and 4 need homogenisation (the metal is more likely to be a mix of solid/liquid as a result)
Overall, are gold alloys easy to cast?
and why? (3)
Easy to cast
Low casting temperature
Low shrinkage (1-4%)
High density
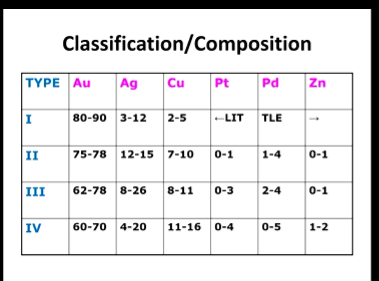
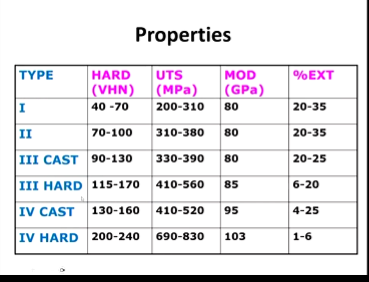
What is the trend in properties as you increase in type
As you increase in type, higher properties
Only type 3 and 4 have Cu > 11% so order hardening is possible hence why type 1 and 2 don’t have order hardening
hard stronger than cast
Type 1 alloys:
Yield stress?
what strengthening mechanisms can oyu do to this?
uses?
Low (this means it can be deformed more easily)
Burnished - improve marginal fit and increases hardness (cold working)
small, well supported inlays where forces are low
Type 2 alloys:
Yield stress?
Strengthening mechanism?
Uses?
Improved properties over type 1, higher yield stress
can still be burnished
used for larger inlays, not in thin sections
Type 3 alloys?
Can they be hardened?
Bonding to adhesive resins?
Can they be burnished?
Yields stress?
Can be hardened (because of the Cu >11%)
Difficult to burnish
High yield stress
Heat to 44 degrees for 10 minutes produces CuO layer which bonds to adhesive resin luting agents
Uses of type 3 alloys?
Inlays/onlays
Full crown and short span bridges
Cast posts and cores
Type 4 alloys
Yield stress?
uses?
can be it used for inlays?
Very high yield stress
High stress situations, partial dentures and clasps, long span bridges
Cannot be burnished so not suitable for inlays
Medium golds:
Composition: (4)
phases?
Au, Pd, Ag, Cu
The added metals all form solid solutions with Au
Single phase structure
Low gold:
Composition: (5)
What colour can they be depending on metal composition?
Phases?
Au, Pd, Ag, Cu, In
Low gold are white
Cu-free low golds containing In are yellow
2 Phases
Ag-Au and Pd-In
Medium/Low golds alloys
price and properties compared to High gold alloys?
Cheaper
Enough Cu to heat harden
Similar properties to type 3 gold

Silver-palladium alloys can be known as?
Composition (5)
Properties?
White gold
Pd, Ag, some Cu, Zn, In
difficult to cast, low density and high Mpt
Tarnish (silver, Pd increases corrosion resistance?)
Cheaper than gold alloys

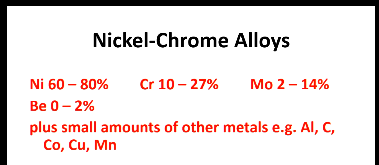
Nickel chrome alloys
Composition: (9)
Ni, Cr, Mo, Be
Small amounts of: Al, C, Mn, Cu, Co
Ni adds what?
Cr adds what?
C?
Ni - strength and hardness
Cr - hardens by solution hardening, corrosion resistance through a passive oxide layer
Must limit C/ carbide precipitation for strength
Downsides of Ni and Be?
Ni - an allergen so could use Co
Be - toxic
Properties of Ni-Cr alloys are comparable to what type of high gold alloy?
Type 3 gold
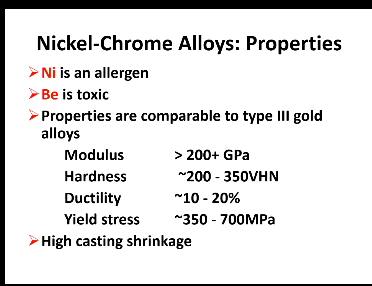
Disadvantage of Ni-Cr alloys?
High casting shrinkage
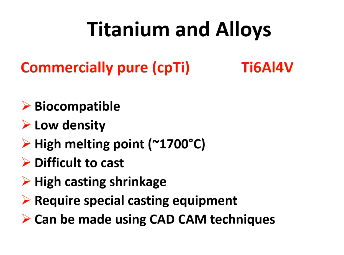
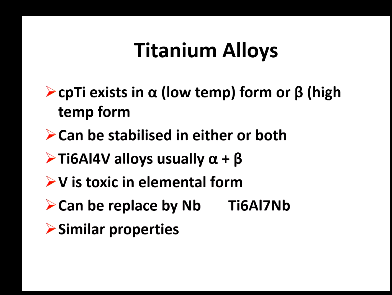
Titanium:
2 types?
Commercially pure and alloy TiAl4V
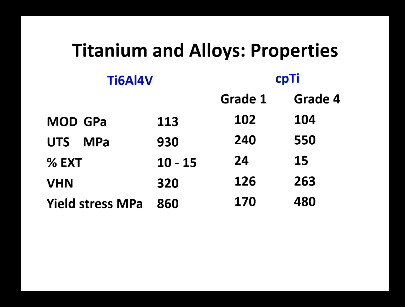
Titanium and alloys properties? (7)
Biocompatible, can be made using CAD/CAM techniques
Low density, high Mpt, difficult to caste, high casting shrinkage, require special casting equipment
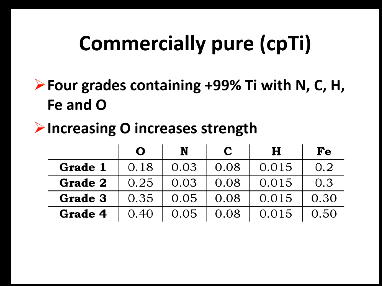
Other elements found in commercially pure Titanium and grades
Commercially pure Titanium exists in what 2 forms
Alpha - low temp
Beta - high temp
In Titanium alloy, what stabilises either phase?
Titanium alloys usually have a mix of alpha and beta
V is toxic in elemental forms and can stabilise
Or V can be replaced by Nb which can also stabilise
Hardness of cpTi and Alloys comapred
PFM?
Porcelain fused metal alloys
Additional requirements of metals in PFM?
Good bond to porcelain
Must not act adversely with the porcelain
Melting range > firing temperature of porcelain
Coefficient of thermal expansion slightly greater than of the porcelain
Low creep or sag (high temp and weight - the metal will permanently deform)
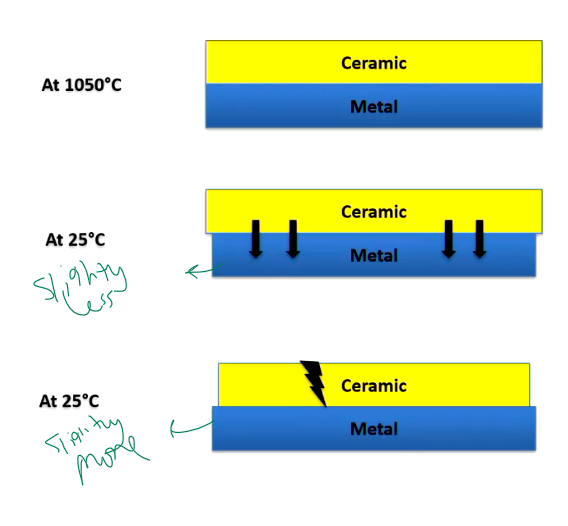
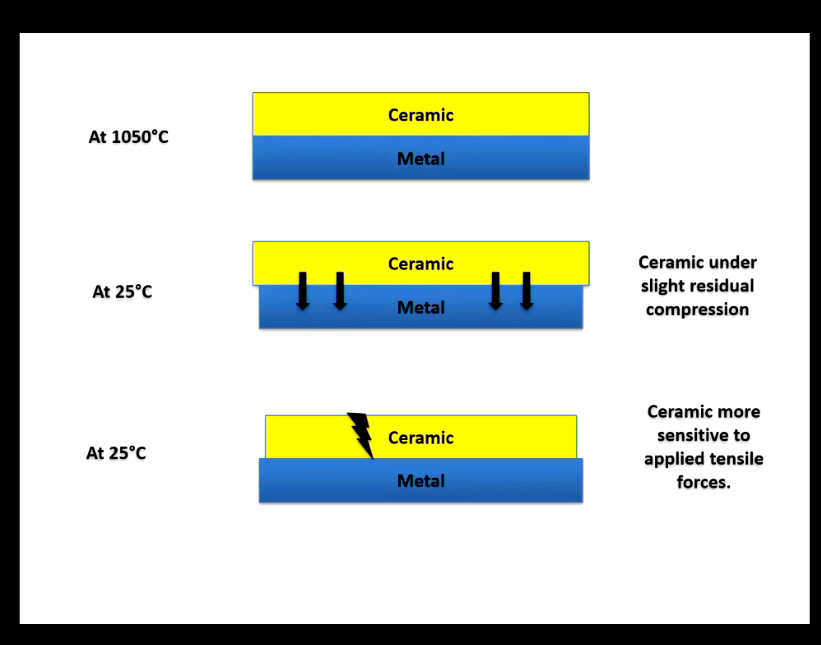
Explain the important of thermal coefficient?
Metal will contract a little more than ceramic, so all forces inwards so ceramic is under slight residual compression (one of the bonding mechanisms of p to m)
Ceramic is under tensile forces, forces are outwards, ceramics are more sensitive to applied tensile forces
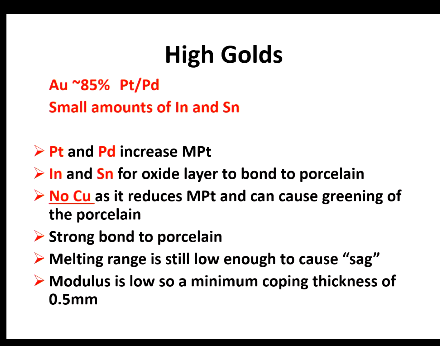
High golds metals used in PFM
What do Pt/Pd DO?
In and Sn?
Cu?
bond to porcelain?
Melting range still causes what?
minimum thickness of coping?
Pt/Pd increase Mpt
Form oxide layer to bond to porcelain
Cu is not include as it reduces Mpt and can cause greening of the porcelain
Strong bond to porcelain
Melting range is still low enough to cause sag
Modulus is low to a minimum coping thickness of 0.5mm
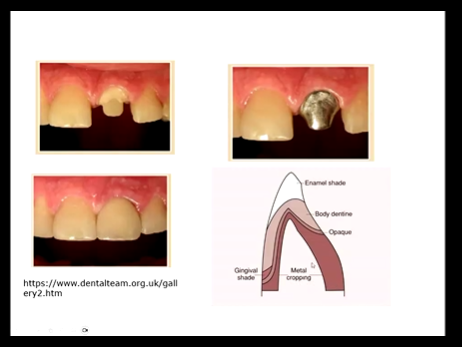
What is a metal coping?
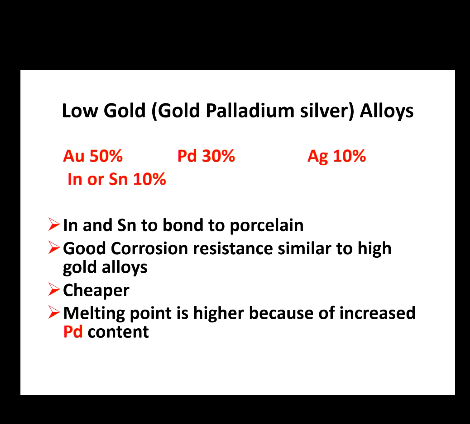
Low gold alloys (Gold, palladium silver) alloys:
What 2 elements allow for bonding to porcelain?
corrosion resistance, similar to?
cost?
Mpt and what element?
In and Sn to bond to porcelain
High corrosion resistance similar to high gold alloy
Cheaper
Melting point is higher because of increased Pd content
Palladium silver alloys
Cost compared to high gold alloys?
castability?
High silver content can cause what?
Offer an alt to high gold alloys at lower cost
Difficult to cast - low density
High Ag content can discolour the porcelain

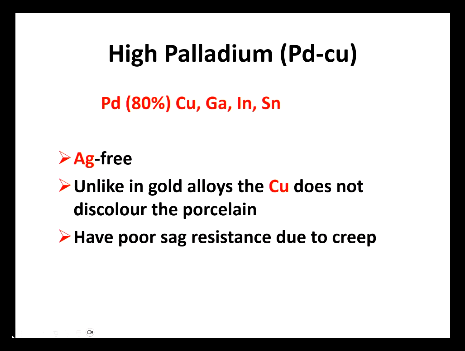
High palladium (Pu-Cu)
is what free?
Cu in this acts differently to when it is in gold how?
creep?
Silver free
Unlike in gold, the Cu does not discolour the porcelain
have poor sag resistance due to creep
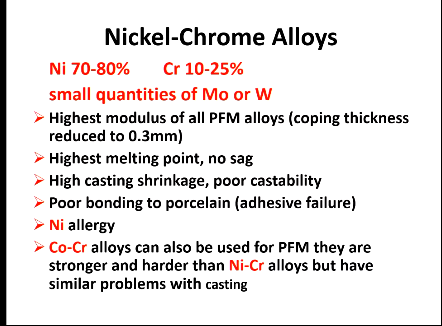
Ni-Cr
Has the highest what compared to other PFM alloys?
coping thickness
melting point
sag
castability?
bonding to porcelain
problem?
alt?
Highest modulus of all PFM alloys
0.3 mm
Highest
No sag
High casting shrinkage, poor
Poor bonding to porcelain - adhesive failure
Ni allergy
Co-Cr alloys can also be used for PFM - stronger and harder than Ni-Cr but still have similar problems with casting
Tilite alloys from Talladium Inc?
manufacturer claims?
Intermetallic compound renders the Ni and Be inert so no allergy or toxicity
Good biocompatibility
good bonding to porcelain
cheaper to gold alloys

Titanium alloys - cpTi and Ti6Al4V?
Mpt?
sag?
bonding to porcelain?
how can cores be made?
Highest Mpt so no sag
Passive oxide layer for bonding to porcelain
cores can be made by CAD/CAM