VIRO 3 | Research Methods for Studying Viruses
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Virus cultivation initially involved _ bc _
Infecting animals
Viruses can only multiply inside living cells
When using animal models as research method for studying viruses, _ must be studied
natural course of infection (pathogenesis = how virus causes disease)
Why must pathogenesis (i.e., process of how a virus causes disease) be studied when using animal models as research method for studying viruses?
Without studying pathogenesis, the animal model may not be able to accurately reflect how virus causes disease bc key aspects, including which tissues are infected, how immune system responds, and what symptoms develop, may be missed or misinterpreted, leading to false assumptions in disease severity, transmission, & treatment effectiveness
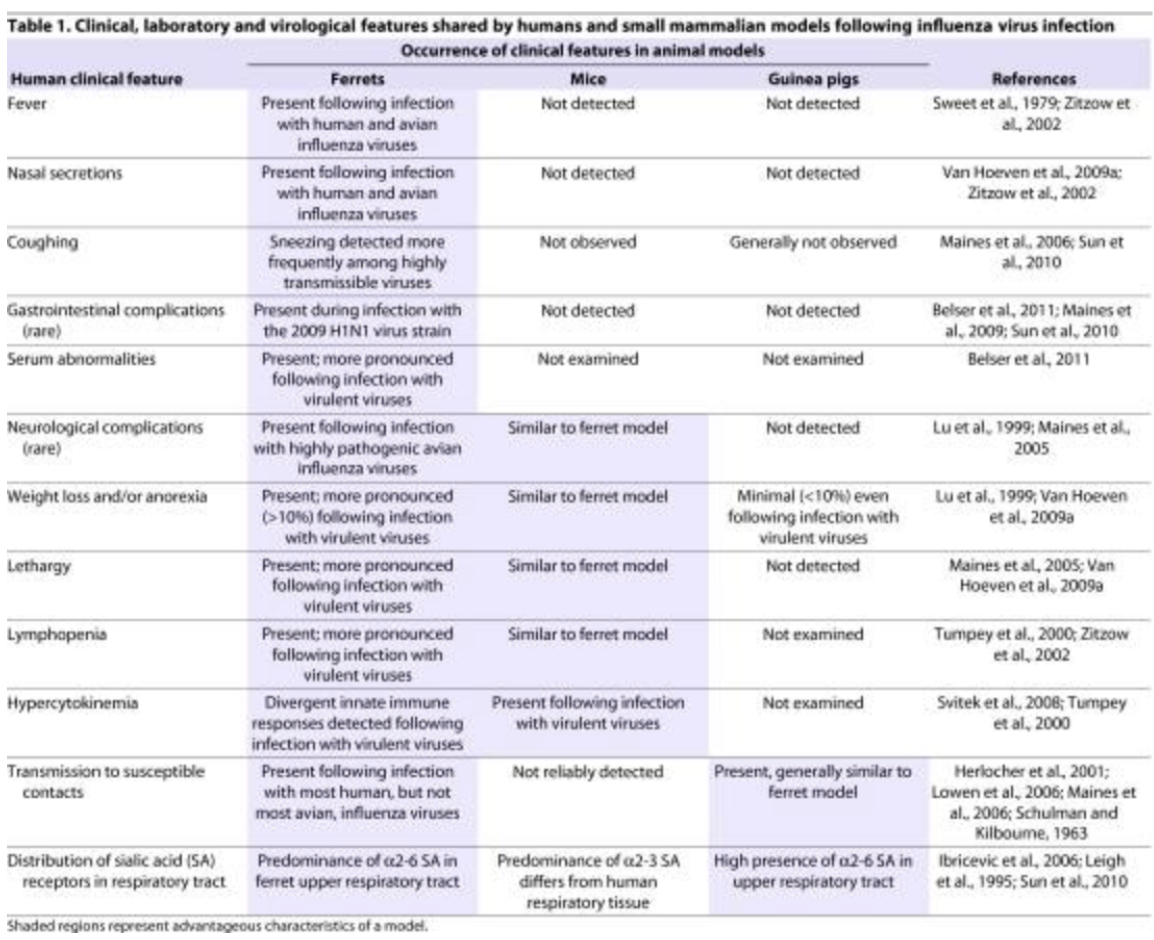
From the 3 animal models (ferrets, mice, guinea pig), _ which share most human clinical features following an influenza virus infection, including _
Ferrets
Human clinical features following IV fnc gstd nw
Fever
Nasal secretions
Cough
Gastrointestinal complications
Serum abnormalities
*shared with guinea pigs
Transmission to susceptible contacts
Distribution of sialic acid (SA) receptors in respiratory tract
*shared with mice
Neurological complications
Weight loss/anorexia
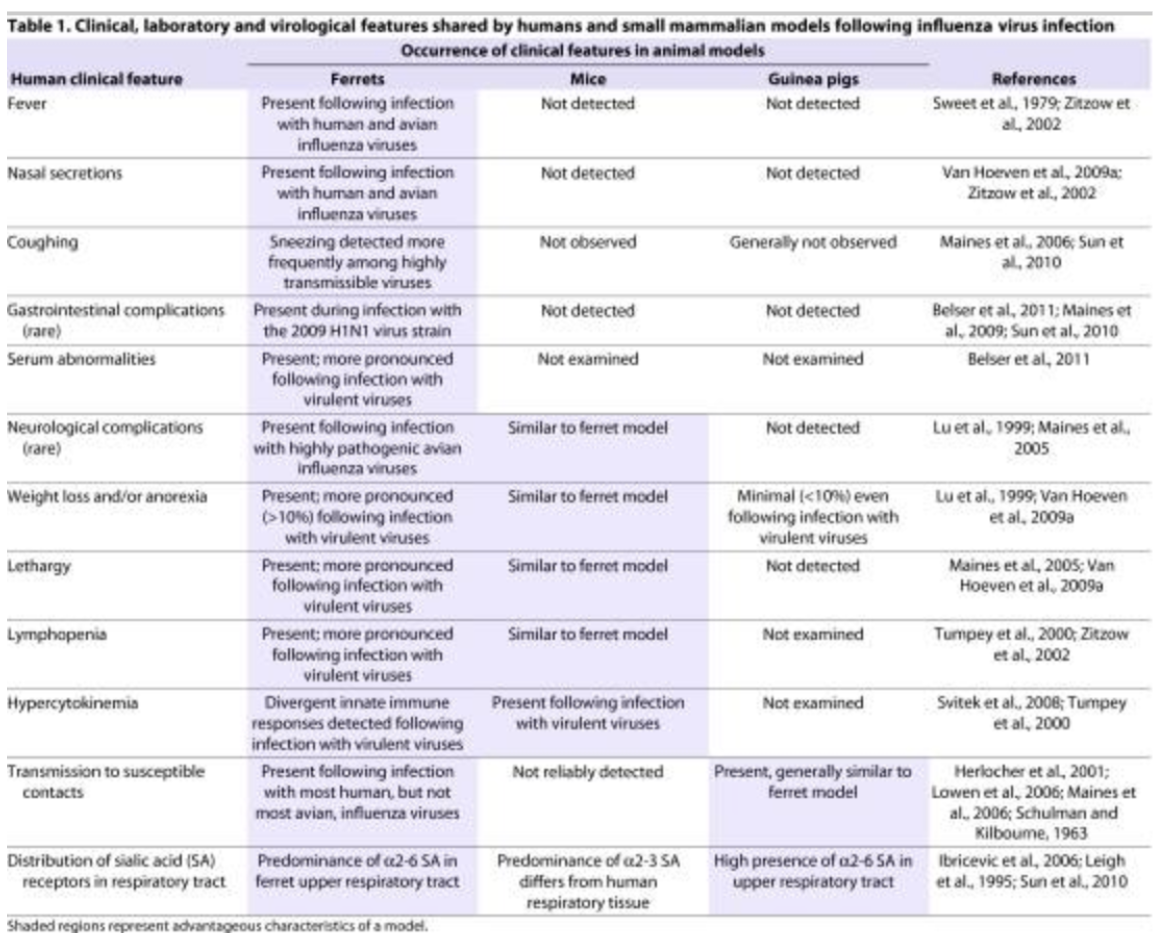
Bc viruses can only multiple inside living cells, another way through which they can be cultivated would be by creating _
embryonated eggs, which is created by poking a small hole on surface of egg shell, inoculating an appropriate tissue with the virus, then sealing it with wax or gelatin
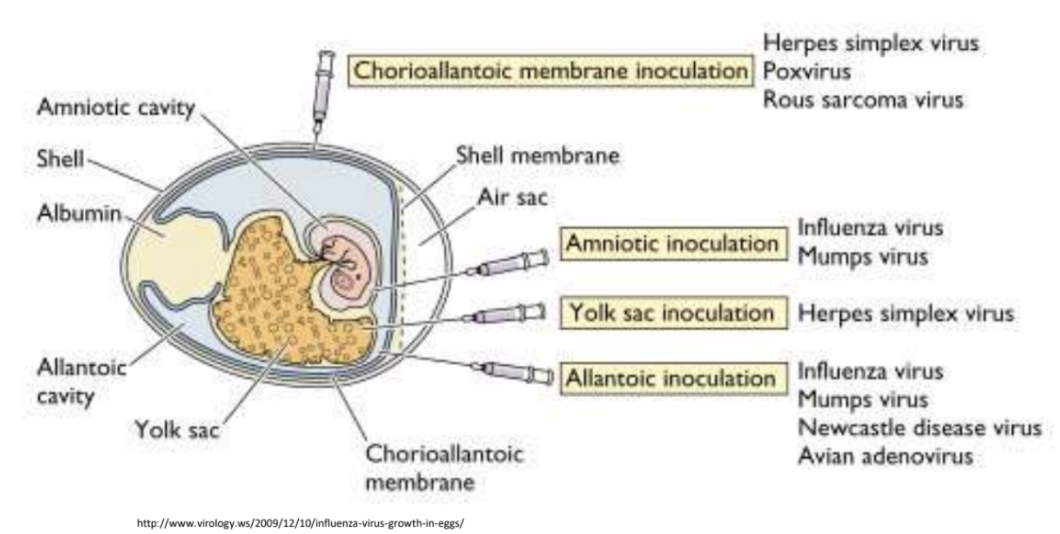
Explain 4 tissues commonly inoculated when cultivating viruses via embryonated eggs
Chorioallantoic membrane hrp
*Rich in capillaries and thus ideal for pock-foming viruses
Herpes simplex virus
Rous sarcoma virus
Poxvirus
Amniotic im
Suitable for isolating respiratory viruses
Influenza virus
Mumps virus
Yolk sac
Nutrient-rich, can support fastidious organisms
Herpes simplex virus
Allantoic imna
Used for high-yield virus production
Influenza virus
Mumps virus
Newcastle disease virus
Avian adenovirus
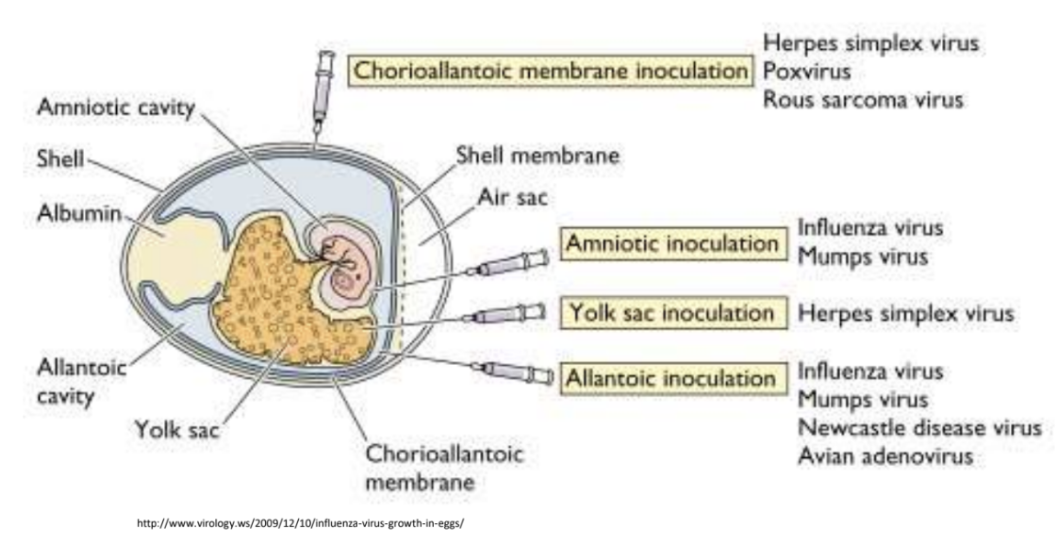
Explain significance of cultivating viruses through embryonated eggs
Influenza virus vaccines are produced through inoculation of embryonated eggs to date, specifically flu viral components, e.g., antigens
*Embryonated eggs at 10-12 days; 1st larger needle punching a hole in shell; 2nd smaller needle inject virus seed into allantoic cavity, followed by 2-3 days incubation

_ refers to the structural & functional changes occurring in host cell due to viral infection
Cytopathic effects
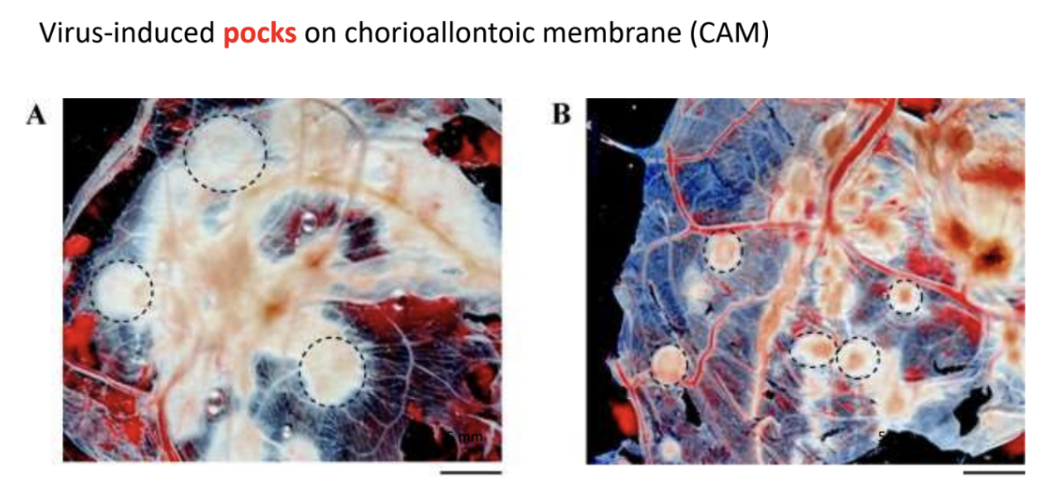
Give examples of cytopathic effects
plmb
Pock marks or lesions on chorioallantoic membrane of egg
Leaf lesions from infection with potato virus Y
Mosaic & chlorosis in leaf lamina
Bumps & ringspots in papaya infected with Papaya ringspot virus

T/F: Initially, observing cytopathic effects was a crude way to quantify virus titer because you can count the pock marks indicative of infectious virions in the solution
TRUE
When an egg is inoculated with virus, _ are observed as cytopathic effects indicative of viral infection
Pock marks or lesions
Except studying pathogenesis, the use of animal models for virus cultivation is generally being taken over by _
faster & cheaper molecular biology methods
Explain 3 choices of virus culture system
Animal culture
Advantage
Natural course of infection
Disadvantages
Upkeep is expensive
Variations between individuals, even if inbred, require large numbers
Ethical considerations
Organ culture (pieces of brain, trachea, gut)
Advantages
Natural infection (mimics site)
Differentiated cell types present
Fewer numbers needed
Less variation since 1 animal gives many organ cultures
Disadvantages
Unnatural since the culture is no longer subjected to homeostatic responses of their immune system
Cell culture
Advantages
Can be cloned and thus variation between individuals is minimal
Good for biochemical studies bc environment can be quickly & exactly controlled
Disadvantages
Harder to study entire pathogenesis
3 types of cell culture
Primary cells = derived from organ/tissue, differentiated but can only survive few passages
Cell senescence is bc of telomere shortening due to cell replication cycles
Cell lines = undifferentiated but are diploid & survive larger numbers of passages (~50)
Bc undifferentiated, they may not fully replicate specialized functions of original tissue
Continuous (permanent) cell line = dedifferentiated but immortal; infecting these with virus can create continuous cell line
What is the significance of the differentiation state of cells in a cell culture system?
Differentiated cells in primary cells = closely mimic the structure & functions of original tissue but can only survive few passages
Undifferentiated cells in cell line = have more proliferative capacity but could lose some specialized functions, making them less representative of in vivo tissue
Dedifferentiated cells in continuous cell line = can divide indefinitely, making these ideal in large-scale / long-term experiments, but would often behave very differently from normal cells due to altered gene expression & abnormal karyotypes
TLDR: The more differentiated, the more representative of original tissue but the lower the survivability; the more dedifferentiated, the more the proliferative capacity but the farthest from being representative of original tissue
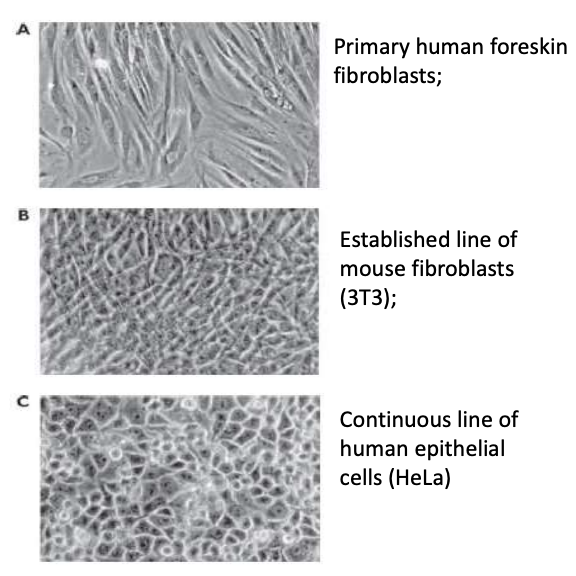
Explain examples of each type of cell culture
Primary human foreskin fibroblast
Cells are derived from embryo / adult organism then cultured under growth medium
Differentiated cells eventually stop dividing due to cell senescence or shortening of telomeres due to replication cycles
After a few passages, differentiated cells (most representative of in vivo tissue) stop dividing
Established line of mouse fibroblast (3T3)
Divide a little longer than primary cells but still have finite number of replications
Undifferentiated = lost SOME specialized functions
Continuous cell line of human epithelial cells (HeLa)
HeLa = first immortalized cell line
Dedifferentiated cells that have undergone transformation via accumulation of mutations or viral infection, which gave them ability to divide indefinitely but also behave differently from original tissue
Used for long-term experiments but do not mimic natural tissue behavior
How are these grown in the lab
Cells form a monolayer at the bottom of culture dish, then immersed in growth medium
Observed under inverted microscope (lens below, light source above) bc cells adhere and thus grow on bottom of plate
Explain extensively all considerations when setting up cell culture systems
Difficulties
One of the difficulties would be avoiding bacterial & fungal contamination
Add antibacterials + antifungals
Add penicillin-streptomycin; BUT BE CAREFUL when adding bc this could overwhelm your animal cells and cause them to die
Apart from growth medium components, fetal bovine serum is also added to provide essential growth factors needed for cell division
Source
Sources of primary cell cultures are usually either duck or mice embryo bc these are rapidly dividing cells
For duck embryo
Get fertilized egg, break it open, obtain embryo, then dissect for tissues you want to culture
e.g., connective tissue, heart cells, fibroblasts, liver, brain cells
On the correct medium, heart cells can still beat as long as provided with calcium in proteins
For mice embryo
Mucus plug in vaginal area indicates successful mating
You count days post-mating, then extract bead-like embryos and dissect them for tissues
Tissue obtained must be macerated & homogenized via
Physical means = blade
Enzymatic method = trypsin (proteolytic enzyme that digests extracellular matrix)
Monolayer formation via contact inhibition, subculturing
Allow culture to adhere to bottom of flask
Eventually, this will grow into a monolayer then experience contact inhibition (when cells run into each other, they stop dividing)
Adhesion cell culture > primary cell culture
If you want to culture more cells, u can passage this after several days to form cell lines (undifferentiated & would lose SOME specialized function but could divide more than primary cells)
Eventually, after several passages, primary cells would no longer divide
OR they could undergo transformation via mutation accumulation / viral infection that allows them to become “immortalized” or divide infinitely but could likely become genetically unique from original tissue culture
Incubation
Cell cultures are usually incubated in CO2 incubator with specific oxygen amount & humidity that must be maintained
Glasswares
Culture disks or flasks are made of special plastics that allow cells to adhere to bottom
Ensure aseptic conditions; work in separate BSCs when working with diff types of cultures (bacteria/fungi/virus)
Scaling up via suspension culture
However, adherent/monolayer culture are only small-scale cultures that can accommodate 20-50 mL
Adherent cultures can only accommodate small volume bc these are limited to available surface area and having small volume allows nutrients & O2 to reach cells more evenly & efficiently
To scale up to liters of culture, you can create suspension culture via trypsinization, which would detach adhering cells on bottom and allow them to float freely within medium
Suspension cultures can accommodate larger volumes bc cells are simply floating freely in medium that could be stirred by bioreactors, thus would have no concern with regards to even nutrient & O2 distribution
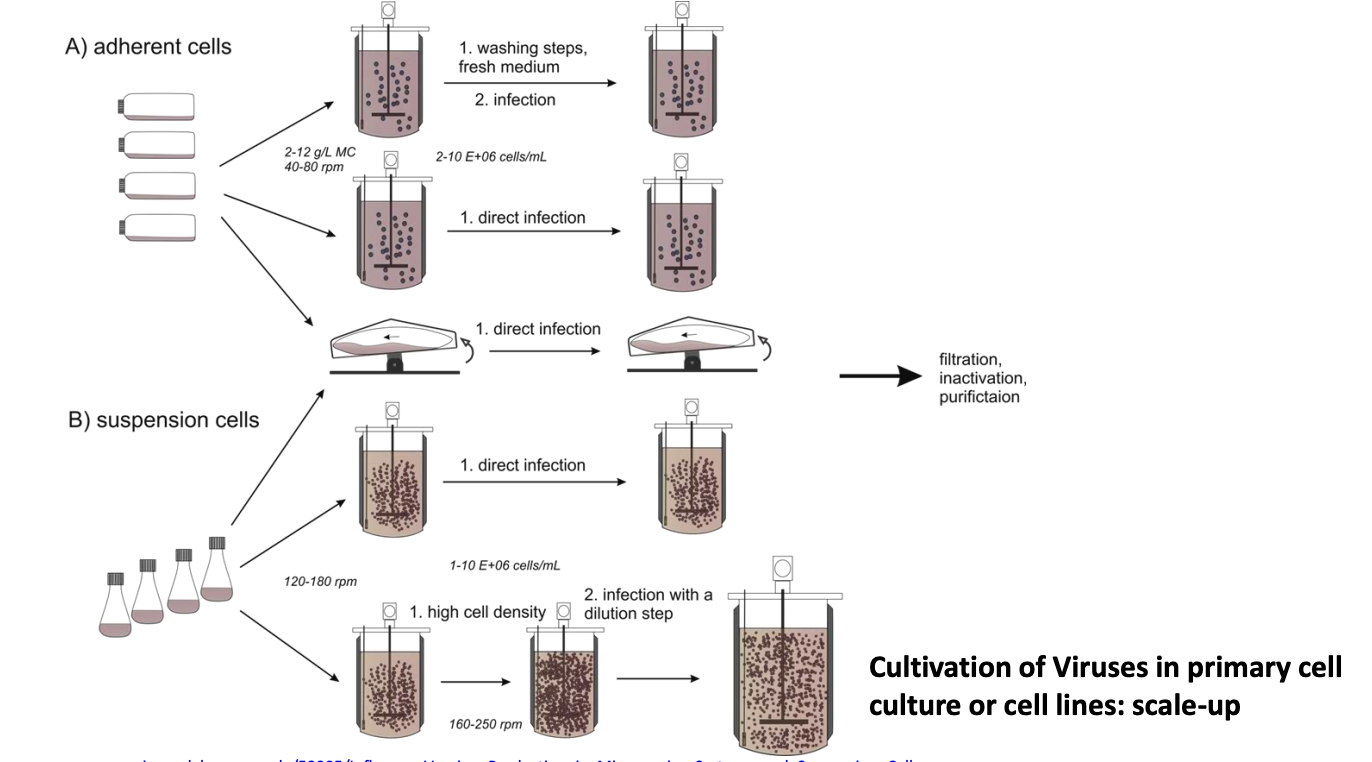
Explain how to scale up virus cultivation using adherent or suspension cells
Adherent cells (cells grow attached to surfaces)
Wash them, give them fresh medium, then infect
Directly infect
Suspension cells (freely floating)
Directly infect
Increase cell density, then infect
Once virus is harvested, these are subjected to fip
Filtration
Inactivation
Purification
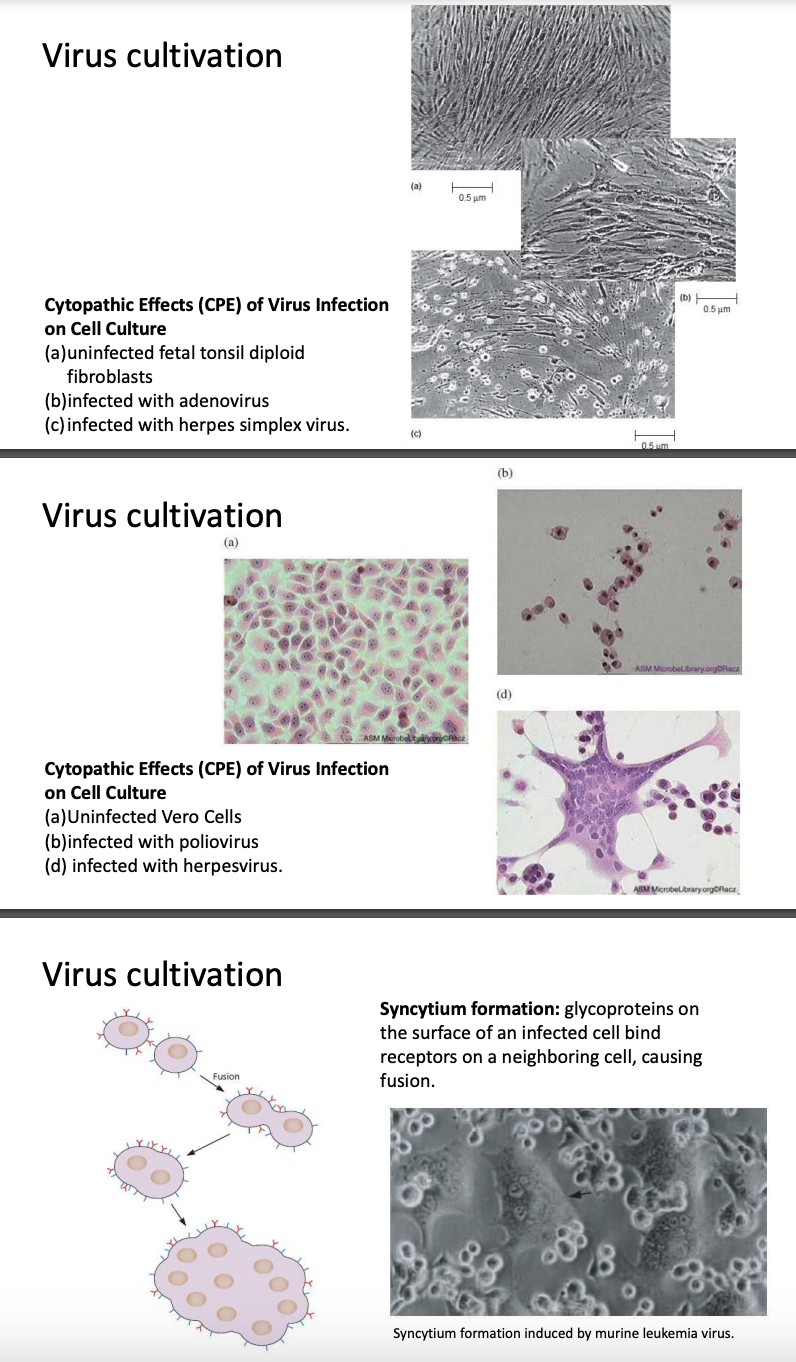
Explain cytopathic effects & adherent cells
Cytopathic effects can also be observed in adherent cells
Fetal tonsil fibroblast
Fibroblasts = connective tissue cells commonly used to make primary cell culture
(B) Fibro infected with adenovirus
(C) Fibro infected with HSV
Different viruses have different cytopathic effects
Not all viruses will produce cytopathic effects
Microscopically, you may not be able to confirm viral infection
Vero cells
(B) Vero cells infected with poliovirus = cells are clumping together & no longer exhibit polygonal shape; not adhering to culture flask anymore
(C) Vero cells infected with herpesvirus = cells are merging and becoming multinucleated, forming a synctium
Synctium formation = glycoproteins on surface of infected cells binds receptors on neighboring cells, causing fusion
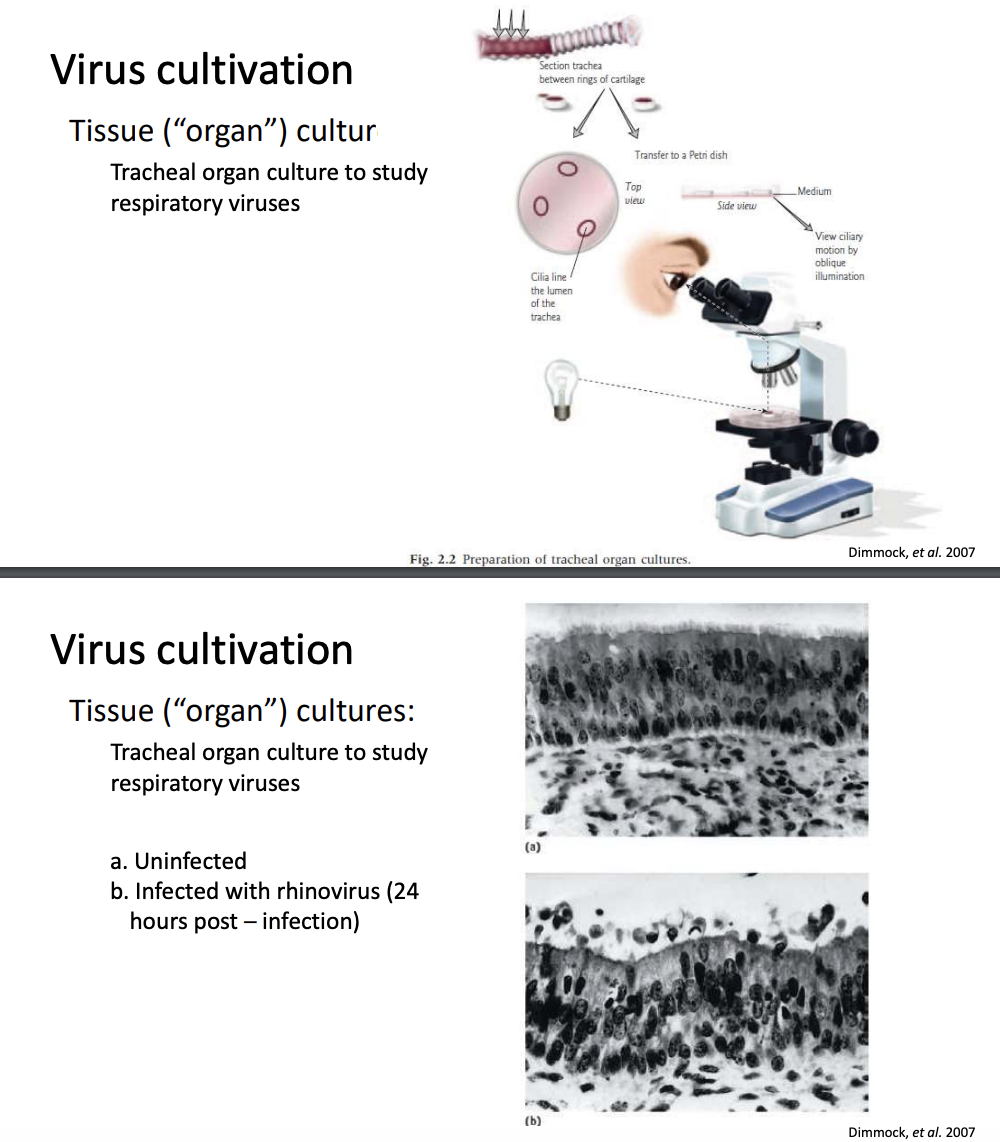
Explain tissue / organ culture
Aside from cell culture, tissue/organ culture may also be used
Tracheal organ culture is most commonly used for studying respiratory viruses
You must keep the intact cell still with cilia on apical surfaces, basal cells
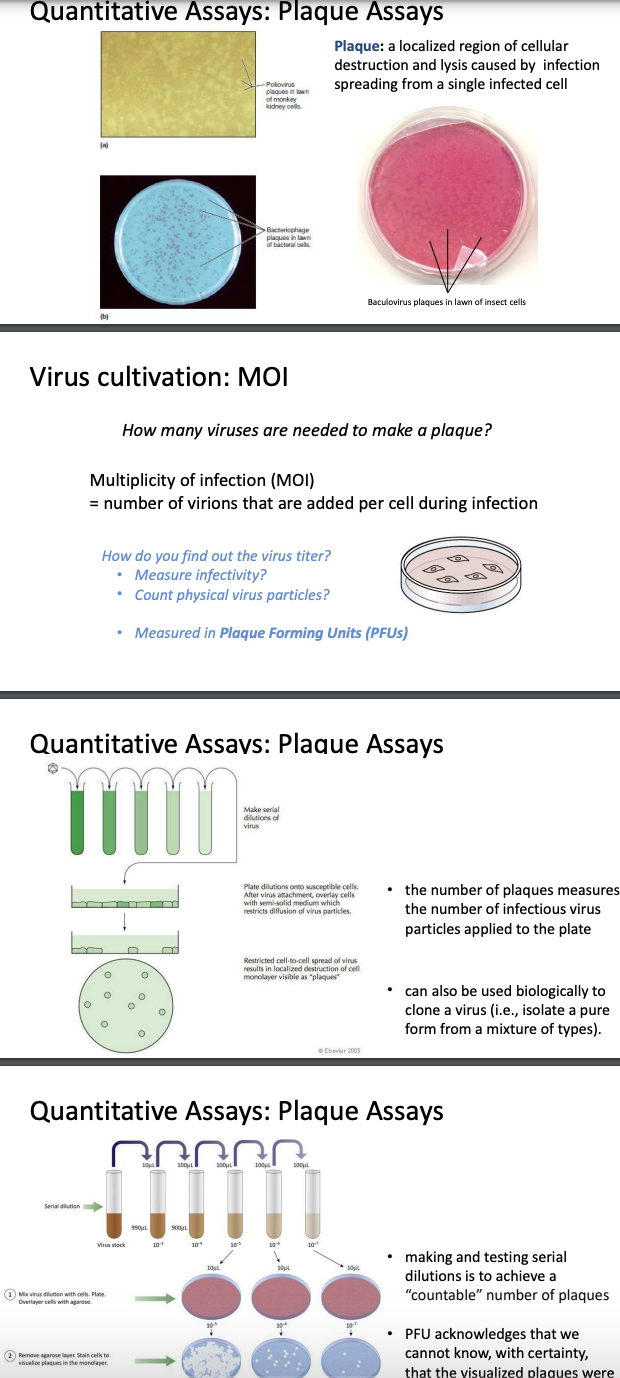
When tissue culture is infect with rhinovirus, it becomes very disorganized
But again this is limited by being unnatural since the culture is no longer subject to homeostatic responses of immune system; cannot study pathogenesis
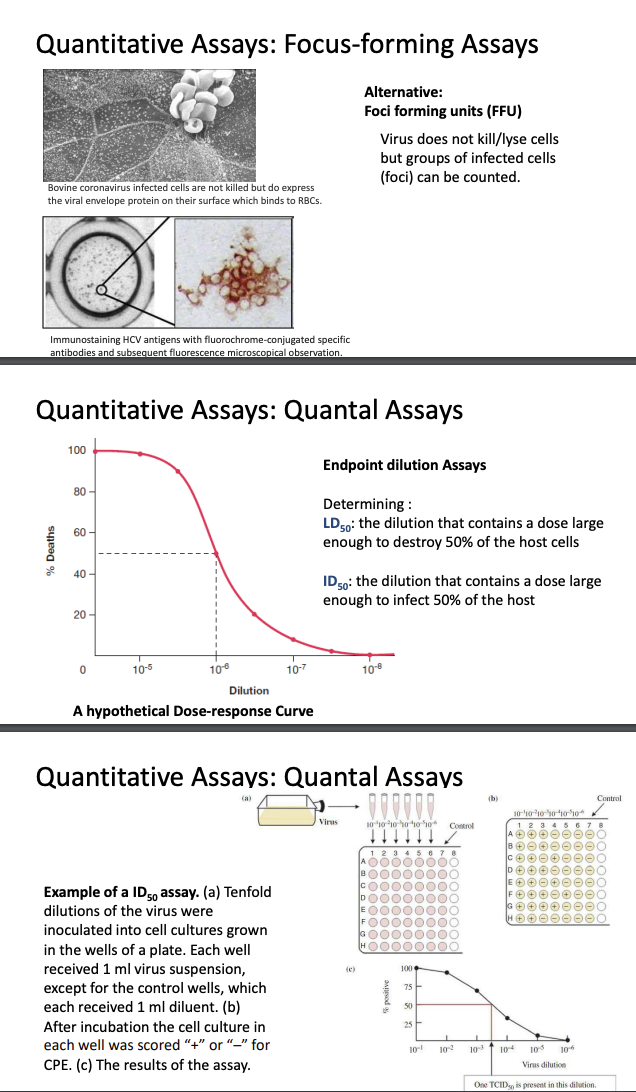
Explain plaque assay
Plaque assay is one way to observe cytopathic effects
Used to estimate virus titer; no. of plaques = no. of infectious virus particles in solution
Used to biologically clone a virus, i.e., isolate pure form from mixture of types
Only applicable for viruses that lyse their host cells
Plaque formation
Plaque = hole in monolayer formed due to 1 cell being infected by a virus; viral replication lyses host cell
Adjacent cells become lysed, forming plaque
Plaque is like a colony; we assume that plaques are formed by the same virus infecting the cell
Number of plaques = depend on virus titer (number of virions present in initial solution)
Multiplicity of infection = average no. of virions added per cell during infection
Not a natural trait of virus but a controllable quantifiable measure that depends on number of virions added; number of cells present
If MOI = 1, then 1 virion is added per cell during infection, BUT this does not necessarily mean that if u have 5 cells and if u add 5 virions, all will be infected
Instead, higher MOI = higher probability of all being infected; lower MOI = lower chances of all being infected (plaque formation)
Serial dilution
e.g., Poliovirus inoculum, monkey kidney cell monolayer
Viral inoculum should be serially diluted; chose the most diluted or the dilution that will allow countable number of plaques
Inoculate diluted virus solution into monolayer culture, then allow virus to adhere
After some time, add semi-solid agarose solution because viruses move freely in liquid medium; thus, overlaying it with semi-solid agarose solution would prevent virus from diffusing all over the plate, preventing formed plaques from overlapping, and forming distinct countable plaques
PFU/mL
Virus titer / original conc. = number of plaques/mL used (DF)
DF = fositive
PFU/mL because it is uncertain whether each plaque formed is formed by a single virion
Plaque assay problem
0.10 ml of a 10-6 dilution of the virus preparation yields 75 plaques. What is the original concentration of PFUs?
PFU/mL = no. of plaques / mL (DF)
= 75 / 0.10 mL (106)
= 7.5×108 PFU/mL
Explain foci-forming units (FFU), quantal assays (LD50, ID50) as other ways to measure groups of infected cells
Foci-forming units (FFU)
Pock marks can be counted as quantitative measure for infected cells
Sometimes, viruses don’t lyse/kill host cells, thus a group of infected cells called foci may be counted
e.g., Bovine coronavirus-infected cells express a viral envelope protein that binds to red blood cells
To quantify infected cells, u can immunostain virus with specific antibody conjugated to chromophore to count foci
Quantal assays (endpoint dilution assays)
Sometimes, you’re not interested in inoculum but in LD50, ID50
LD50 = dilution with dose large enough to kill 50% of cells
ID50 = dilution with dose large enough to infect 50% of cells
Setup = 10-fold dilutions of virus inoculated into cell cultures grown in wells; 1 mL of virus suspension is inoculated into each well; after incubation, wells are marked with ± for CPE
Dose response curve = useful for determining dilutions needed to kill/infect a certain % of cells
Y = % of dead/infected cells
X = dilutions of virus suspension
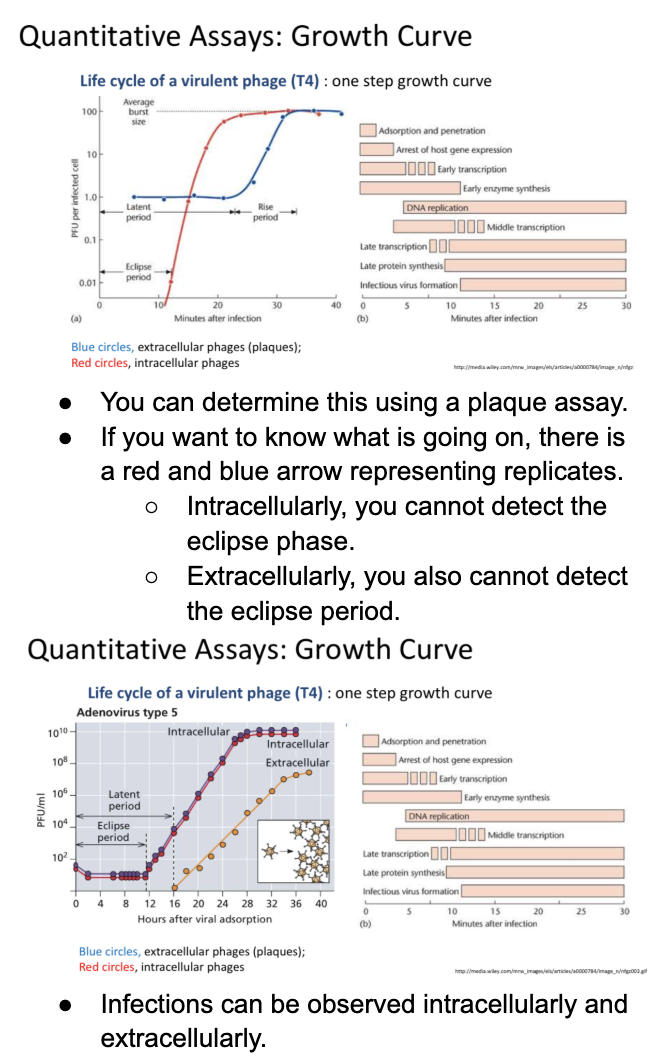
Explain growth curve quantitative assay as another way to measure group of infected cells
Bacteria = cell numbers over time
Optical density = limited bc there could still be dead cells in there
To count live cells, plate inoculum
Viruses = abrupt
One-step growth curve
Eclipse period = time until the viruses are assembled and thus detected
Latent period = time until viruses are released from infected cells
When cell is infected with virus,
undergoes eclipse phase = where no viruses are detected bc there is no virus assembly yet
even if virus has entered host cell & lysed it; lysate is used to infect other host cells > no assembly of virions yet
Log phase = occurs when virions have been assembled
Followed by stationary phase = release of all virions
One-step vs. Two-step: which would have lower MOI?
One-step = higher MOI bc this means that it only took 1 burst for all cells to be infected, implying that there were sufficient virions to infect all cells
Two-step = lower MOI bc first burst released virions not enough to infect all cells, hence 2nd burst was needed to infect remaining viable cells
Can be determined using plaque assay
However, eclipse phase cannot be detected intracellular & intracellularly
Virions are only detected intracellularly after assembly; while virions are detected only extracellularly after release from infected cells
Explain hemagglutination & ELISA as other ways to measure no. of infected cells
Hemagglutination
Some viruses agglutinate RBCs bc they bind to proteins on RBC surface
If virus agglutinates RBCs, mixture spreads into a lattice at bottom of well (+)
If virus doesn’t, RBCs will settle as tight button at bottom of well (-)
Hemagglutination titer = reciprocal of highest dilution that shows positive agglutination results; as virus becomes more diluted, it loses the ability to agglutinate
Highest dilution = 1/128
Hemagglutination titer = 128
Enzyme-linked Immunosorbent Assay (ELISA)
Enzyme-linked = conjugation of antibody with reporter enzyzmes
Immuno- = antibody-antigen interactions
-Sorbent = adsorption of antibody/antigen
Purpose
To detect viral antigens/host antibodies
Color change = binding / (+)
Setup components
Antigen/antibody is adsorped to plate
Blocking agent (skim milk) is added to prevent nonspecific binding
Primary antibody/antigen binds to target antibody/antigen
(Indirect) Secondary enzyme-linked antibody binds to conserved region of primary antibody
Add substrate > color change > (+)
Indirect ELISA = detects host antibodies
Coat plate with viral antigen
Add blocking agent then patient serum with antibodies
Target antibody binds to antigen
Secondary enzyme-linked antibody binds to conserved region of primary target antibody
Substrate > color change
Common for serological testing
Direct ELISA = viral antigens
Target antigens adsorp to plate
Enzyme-linked antibody binds to target antigen
Substrate > color change > (+)
Sandwich ELISA = low-level viral antigens
Coat plate with primary capture antibody
Target antigens bind to capture antibody
Secondary enzyme-linked antibody binds to different epitope of the same target antigen
Substrate > color change > (+)
Considerations
COVID-19 = lateral flow antigen test (ELISA principle)
HIV = direct virus detection is hard; detecting viral antibodies (indirect ELISA) is preferred
2-antibody system = cost-efficient bc generic secondary metabolites can be reused; no need to label every primary antibody bc universal 2ndary antibody
Explain NAATs as viral detection techniques
Nucleic Acid Amplification Techniques (NAATs)
PCR
Template = DNA
To amplify specifc DNA sequences using Taq polymerase, which has no proof-reading abilities and prioritizes speed > accuracy
High-fidelity amplification = PFU / PMX polymerase
Important if u plan to express DNA product later
RT PCR
For viruses with RNA
Reverse transcriptase converts RNA to cDNA
DNA polymerase synthesize dsDNA
High-Throughput Sequencing
Enables analysis of multiple samples at once from amplification to sequencing
Useful for vef
Virus population genomics (insights into how RNA viruses could have different sequences in an individual - quasispecies)
Evolutionary biology & outbreak tracing = u can look at outbreaks & figure out spatial relationship between outbreak & metagenomics (environment-derived genetic material)
Phylogenetic analysis = phylogenetic trees can show reinfection & lineage relationship of SARS-CoV-2
Detecting viruses via metagenomic analysis
Analyzes environmental / biological samples without isolating individual viruses
Samples: Soil, oceanwater, human tissue, wastewater
Key techniques
DNA/RNA extraction
PCR / RT PCR
Sequencing
Identifies tvf
Taxonomic profiles = which viruses are present
Virulence factors = assess functional potential & pathogenic risk of microbial community
Functional genomics = what each gene does
Real world example
During COVID-19, SARS-CoV-2 RNA was detected in wastewater
Sick individuals excreted viral particles into wastewater system
Wastewater was analyzed to ets
Estimate community viral load
Track prevalent strains
Support public health surveillance, contributing to early detection & monitoring emerging outbreaks before clinical testing is widespread
Explain viral diagnostics
Targets for viral diagnostics
Viral proteins/antigens = detect via immunoassays using naturally occurring or recombinant antibodies
Viral genomes = NAATs
Antiviral antibodies = Indirect elisa; detects only exposure, not necessarily active infection
Diagnostic test qualities
Sensitive = should detect even small infections
Specific = prevent false positives
Rapid = to provide quick results
e.g., Lateral Flow Immunoassay (Rapid Antigen COVID-19 Test kit)
Nitrocellulose adsorbent membrane carries sample via capillary action
Sample pad = where u put sample
Conjugate (Primary antibody) pad = binds viral antigens present
Test line = immobilized labeled secondary antibody binds to different epitope of same antigen
(+) = red
Control line = to see if test works; red = valid if it binds unbound labelled primary antibodies
Samples
Blood = detecting antibodies
Nasal swab = detecting antigens
Antibody tests = exposure not active infection
Antigen tests = active infection
Gold Standard = RT-qPCR
Amplifies & quantifies viral RNA
Cycle threshold (Ct) = cycle number at which viral NA become detectable
Lower Ct = more virus particles present
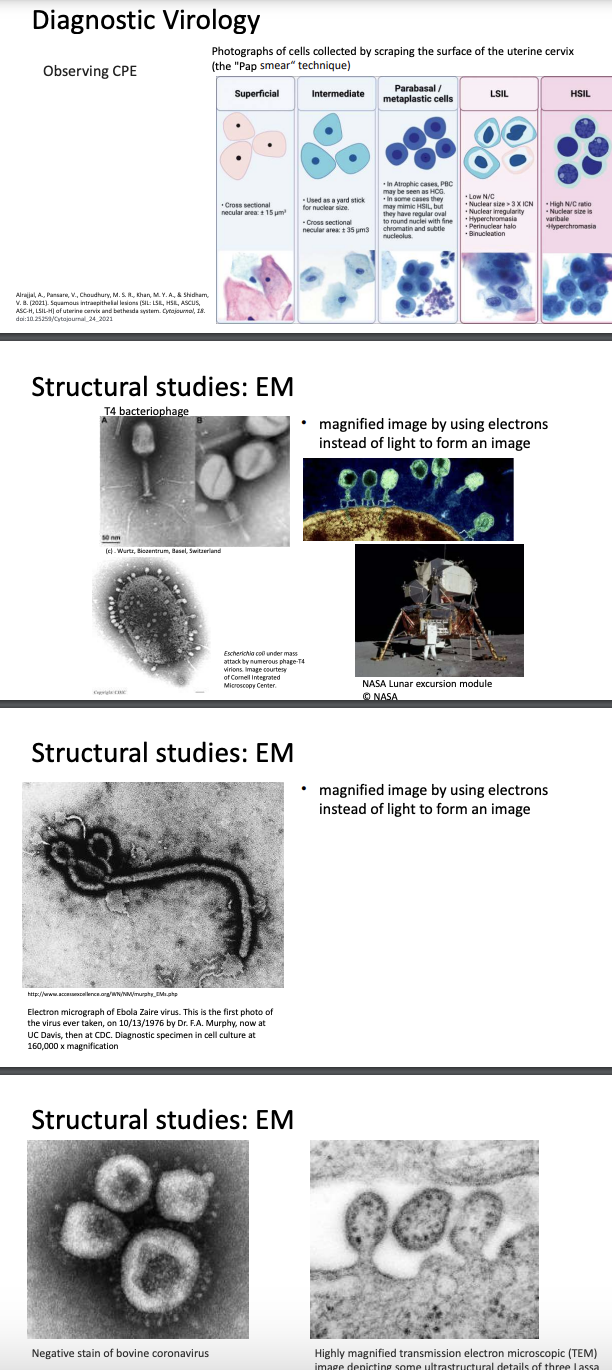
Explain other methods of viral diagnostics (Pap smear, EM)
CPE & Pap smear
Pap smear = used to detect cervical cancer caused by Human Papillomavirus (HPV)
In this method, epithelial cells (superficial, intermediate, parabasal, LSIL, HSIL) are observed under microscope
For HPV, CPE manifests as uncontrolled cell division, which may lead to tumor formation
Electron microscopy (EM)
Uses electrons instead of light to form image
How it works
Beam of electron passes through magnets & is focused on specimen
Specimen scatters electrons & scattered electrons are detected by scanner
Computer then interprets these signals into high-res image
Transmission EM
Uses wide electron beam
Produces 2D image
Visualizes internal structures of virus
Scanning EM
Uses fine electron beam
Produces 3D image
Only shows surface structure of virus
Limitations
Requires sample fixation = samples need to be preserved & dehydrated
Biological samples don’t scatter electrons well; have to be coated with thin metallic layer (gold) to be visualized
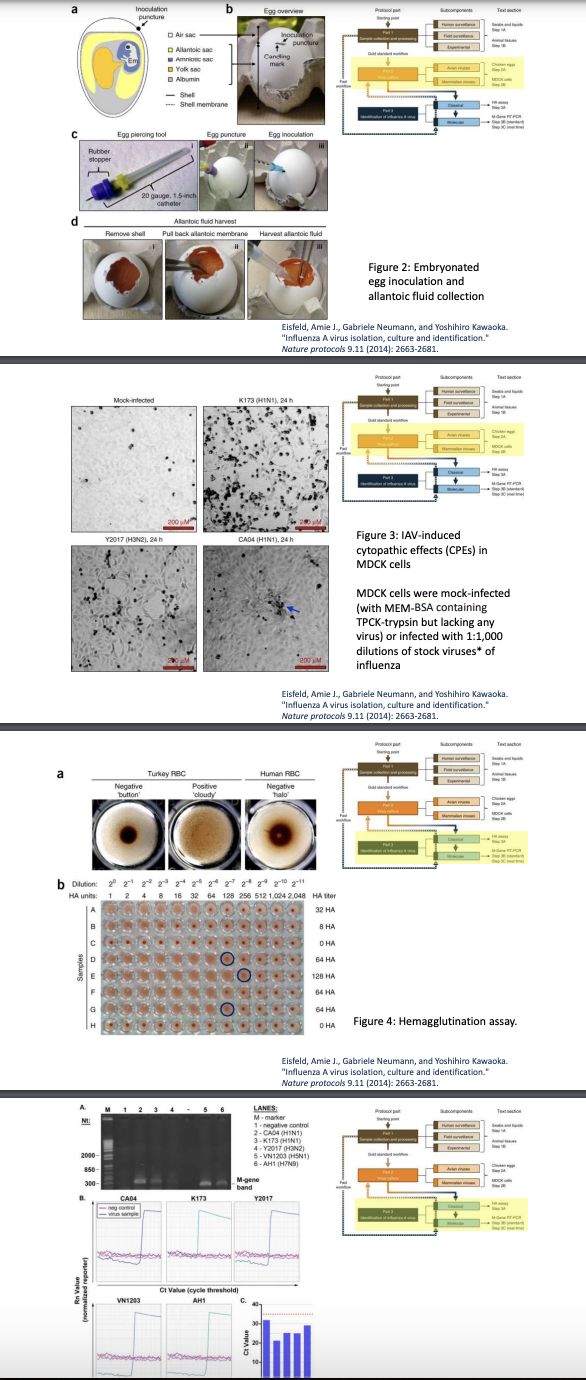
Explain summarized Protocol for Isolating Influenza A Virus
Sample collection & processing
Human surveillance (swabs & liquids)
Field surveillance (animal tissues)
Experimental source
Virus culture
Embryonated chicken eggs
Mammalian cell culture
Identification of Influenza A virus
Classical = HA = uses RBCs to detect presence of viral hemagglutinin
Molecular = RT-PCR = amplifies viral RNA