Honors Chemistry Chapter 8 Study Guide
^^Terms/Things to Know^^
Bonds
^^ionic bonds^^: metals + nonmetals. Electrons of the metal donate to the non mental. These compounds have a high melting and boiling point
^^Metallic bonds^^: metal + metal, structured by delocalized electrons
^^Covalent (molecular) bonds^^: nonmetal + nonmetals. The atoms share electrons in order to achieve an octet. They have low in temperature melting points and boiling points.
Molecular Compounds
- ^^Molecule^^: a neutral group of atoms joined together by covalent bonds. Ex. Oxygen Molecules
- ^^Diatomic Molecule^^: a molecule consisting of two atoms. Can only be true for atoms included in Br I N Cl H O F (Bromine, Iodine, Nitrogen, Chlorine, Hydrogen, Oxygen, Fluorine). Ex. Br2, Cl2, O2
- ^^Molecular Compound^^: a compound composed of molecules. Ex. H20 (Water) and Carbon Monoxide (CO)
- ^^Formula Unit^^: the base of an ionic compound NaCl is an ionic compound so the formula units are Na and Cl. It is an ionic compound because it is made up of an array of sodium ions and chloride ions which is why it isn’t molecular.
- ^^Molecule^^: base unit of a molecular compound. The base unit of water is one molecule of H20.
- ^^Molecular Formula^^: a chemical formula of a molecular compound that shows how many atoms of each element a molecule contains. Chemical formula of water is H20 (two hydrogens and one oxygen).
^^Covalent Bonding^^
The Octet Rule: electron sharing usually occurs so that the atoms attain the electron configurations of noble gases (each having eight valence electrons)
Covalent Bond: joins two atoms together that are sharing a pair of electrons
Lone Pair: a pair of valence electrons that is not shared between atoms. Also called an unshared pair or a non bonding pair.
Atoms form double or triple covalent bonds if they can attain a noble gas structure by sharing two pairs or three pairs of electrons
Double covalent bond: a bond that involves two shared pairs of electrons
Triple covalent bond: a bond formed by sharing three pairs of electrons
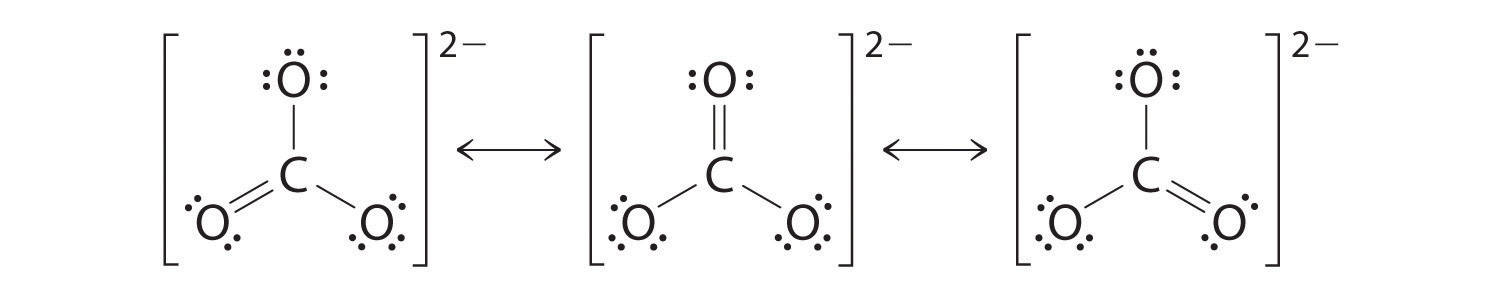
^^Resonance Structures^^
some molecular compounds’ Lewis Dot Structures can be drawn different ways. This is called a Resonance Structure and is typically indicated by a double bond or triple bond in which their locations can be switched.
Example:
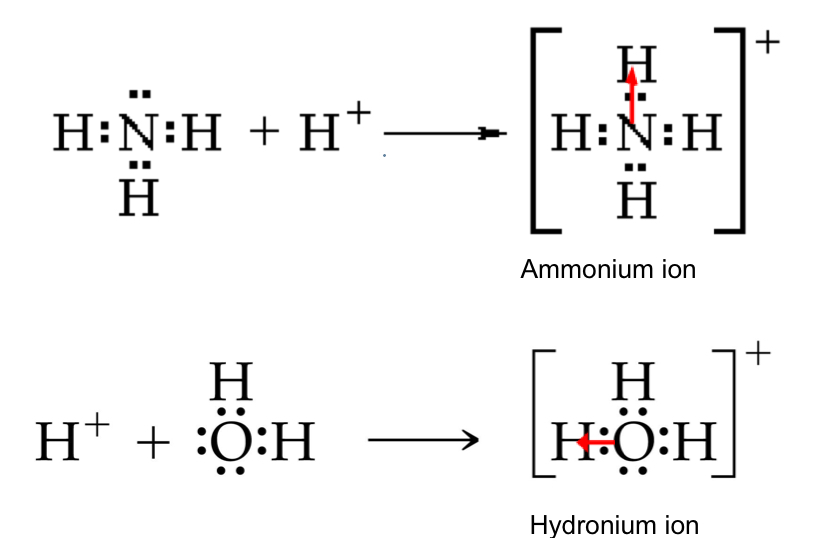
^^Coordinate Covalent bond^^: a covalent bond in which one atom contributes both bonding electrons
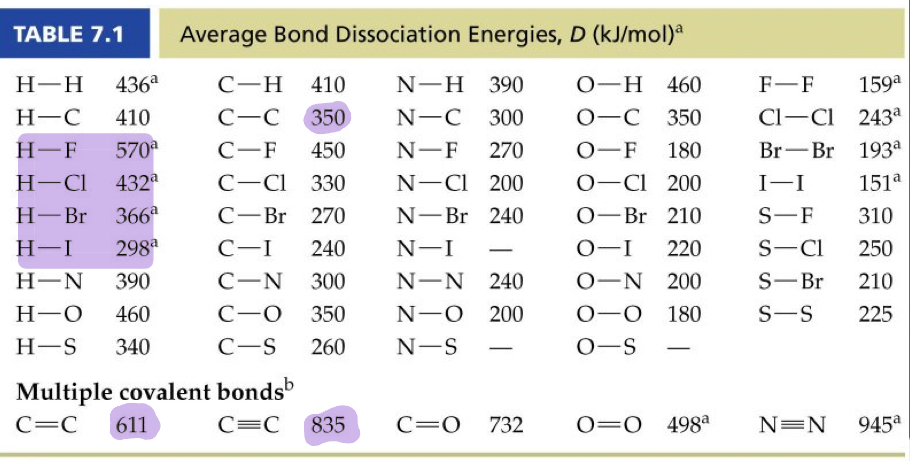
^^Bond dissociation energy:^^ The energy required to break the bond between two covalently bonded atoms. A large bond dissociation energy corresponds to a strong covalent bond.
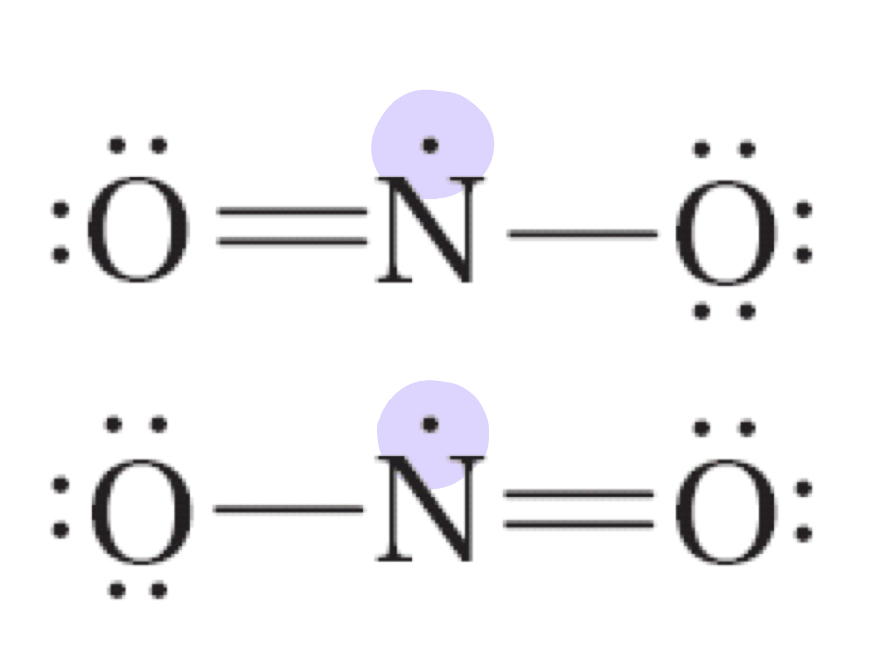
^^Exceptions to the Octet Rule^^
the octet rule cannot be satisfied in molecules whose total number of valence electrons is an odd number. There are also molecules in which an atom has fewer, or more, than a complete octet of valence electrons.
Nitrogen dioxide (NO2) has an odd number of valence electrons and therefore does not satisfy the octet rule
Boron Trifluoride (BF3) can be written so that boron only has 6 valence electrons
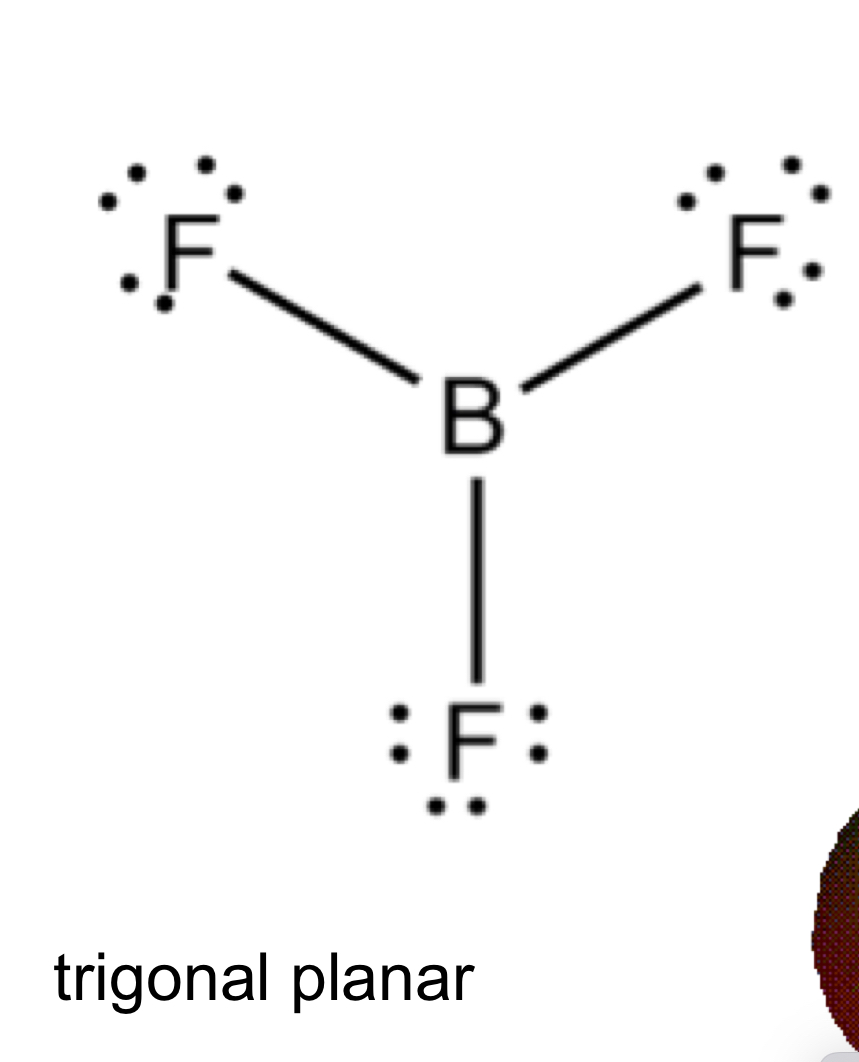
Phosphorus pentachloride (PCl5) can be written so that phosphorus has ten valence electrons
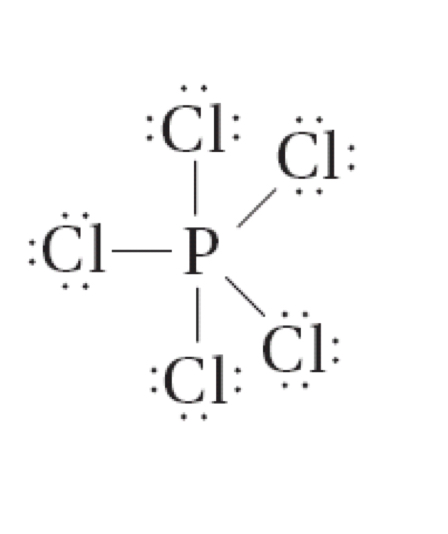
Sulfur hexafluoride (SF6) can be written so that sulfur has ten valence electrons.
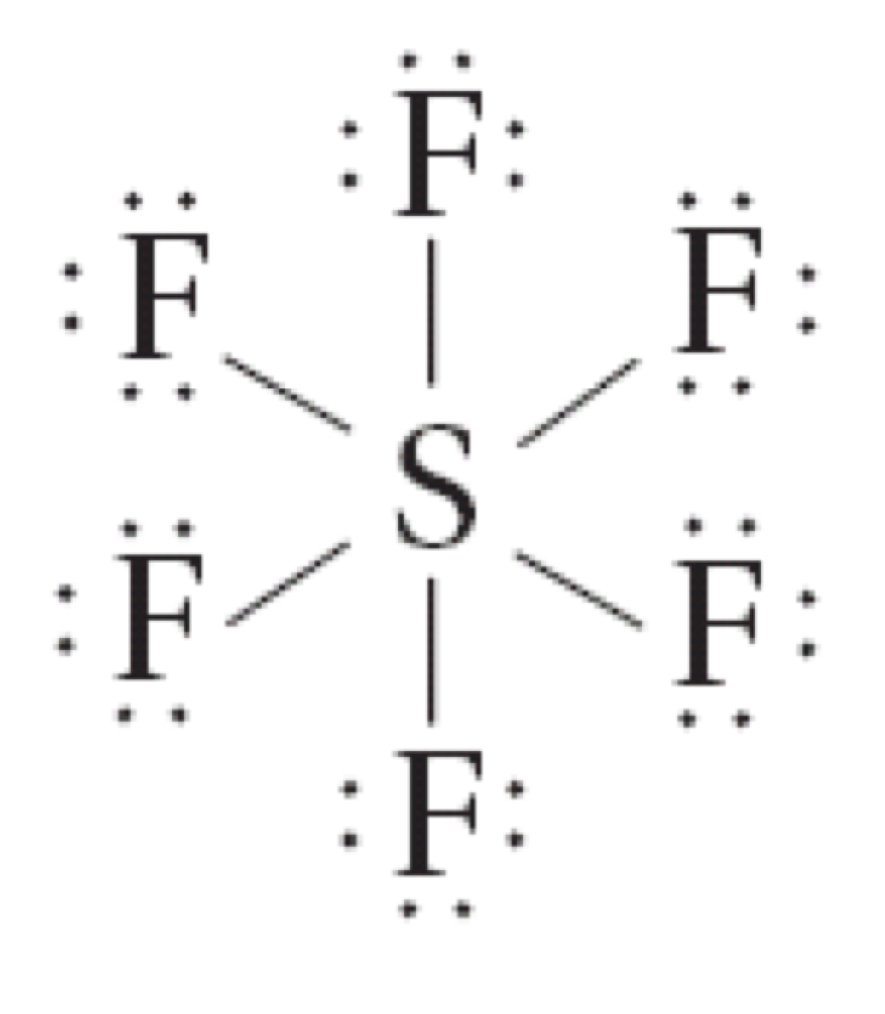
Iodine heptafloride (IF7) can be written so that sulfur has twelve valence electrons.
The structures that possess more than an octet are able to due this due to an expansion to the d-sub shell.
^^VSEPR Theory (valence shell electron pair repulsion):^^
According to the VSEPR theory, the repulsion between electron pairs causes molecular shapes to adjust so that the valence-electron pairs stay as far apart as possible. It is the theory that explains why the molecules form three-dimensional shapes.
The Shapes
| Tetrahedral | 109.5 degrees | 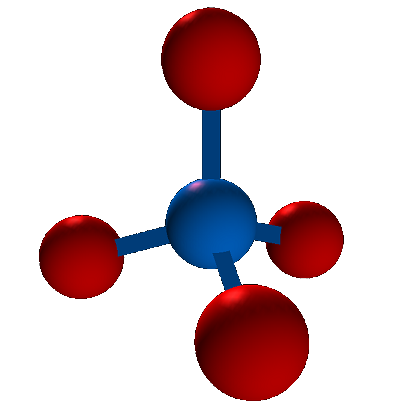 |
|---|---|---|
| Trigonal Pyramidal | 107 degrees | 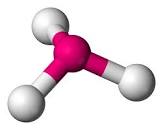 |
| Trigonal Planar | 120 degrees | 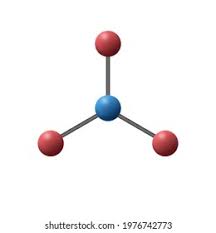 |
| Bent or Angular | 104.5 degrees | 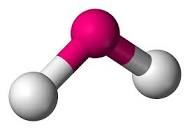 |
| Linear | 180 degrees | 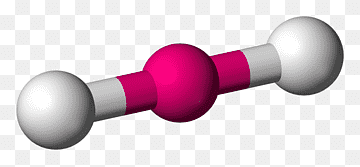 |
Bond Polarity
- ^^Nonpolar covalent bond^^: when the bonding electrons are shared equally. Also called a pure covalent bond. Shown when through a symmetrical molecular structure
- ^^Polar covalent bond^^: the electrons are shared unequally. Shown through an asymmetrical molecular structure.
^^The more electronegative atom attracts more strongly and gains a slightly negative charge. The less electronegative atom has a slightly positive charge.^^
| Electronegativity difference range | Most probable type of bond |
|---|---|
| 0.0-0.4 | Nonpolar covalent |
| 0.4-1.0 | Moderately polar covalent |
| 1.0-2.0 | Very polar covalent |
| 2.0 and on | Ionic bond |
^^Polar Molecules^^
- Dipole: in a polar molecule, one end of the molecule is slightly negative and the other slightly more positive. A molecule with two poles is called a dipolaire molecule of dipole
^^Molecular Orbitals^^
molecular orbitals: orbitals that apply to the entire molecule when two atoms combine and their orbitals overlap
Bonding orbital: a molecular orbital that can be occupied by two electrons of a covalent bond
Sigma bonds: formed when two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis and connects two atomic nuclei. The first bond formed
Pi bonds: the second bond area formed in a double bond. The two are pretty much perpendicular to each other
Hybridization
- ^^hybridization^^: several atomic orbitals mix to form the same total number of equivalent hybrid orbitals because their bond lengths are identical and their bond energies are identical
Attractions Between Molecules
^^inter^^: between.
^^Intra^^: within
^^Intermolecular forces (IMF)^^: weaker than either ionic or covalent bonds but are responsible for determining the state of matter a molecular compound is at a given temperature and a molecule’s melting point and boiling point.
- ^^Vander Waals Forces^^: attractions between the molecules that are the two weakest attractions between molecules
- ^^Dipole/Dipole interactions^^: when polar molecules are attracted to each other
- ^^London Dispersion forces^^: the weakest of all molecular interactions. Nonpolar and caused by temporary asymmetrical dispersion of electrons around it. They are in all molecules but usually more insignificant in comparison to other IMFS. The strength increases as the electron # increases so the bigger (more atoms and mass) the stronger the London force is
- ^^Hydrogen bonds:^^ when a hydrogen atom bonds to a very electronegative atom (Nitrogen, Oxygen, or Fluorine). It is only for polar and are extreme dipole dipole interactions. It is the strongest of these bonds.
^^Network Solids^^
- network solids (or network crystals: solids in which all of the atoms are covalently bonded to each other. The molecules inside do not melt until the temp. Is 1000 Celsius or higher, some don’t melt at all and just vaporize to gas. Ex. Diamond