L6-Polysaccharides
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Glucose is soluble in water. It is soluble because it is a _1? molecule as it is a polar molecule with hydroxyl groups (OH).
This means it can move in and out of cells and effect water _2?.
So it needs to be stored in other forms.
1.Hydrophilic. 2.potential
Polysaccharides are formed by the condensation of many glucose units.
State 3 polysaccharides and how they are formed?
• Glycogen and starch are formed by the condensation of many a-glucose molecules.
• Cellulose is formed by the condensation of many B-glucose.
• Starch- long chain of two polysaccharides (amylose and amylopectin- a glucose)
Starch is the storage polysaccharide of _1?.
It is stored as _2? (starch grains).
Starch is constructed from two different polysaccharides: _3? and _4? (both from alpha-glucose).
1.plants. 2.granules. 3.amylose. 4.Amylopectin
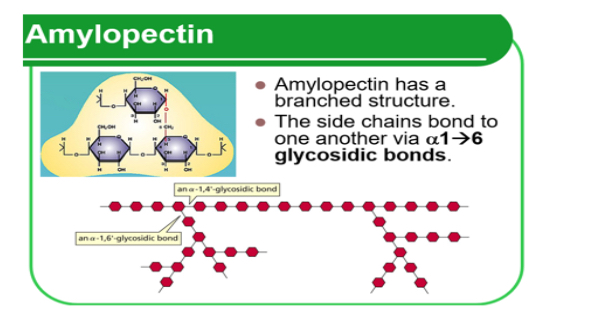
Amylopectin is:
• Polymer of a- _1?.
• Has 1 → 4 glycosidic bonds which forms _2? chains.
• BUT there is a branch on every 25-30 glucose molecules. This bonds as a 1 → 6 _3? bond
• Branching means there are glucose molecules at the ends. These are easily _4?.
1.Glucose. 2.long. 3.glycosidic. 4.hydrolysed
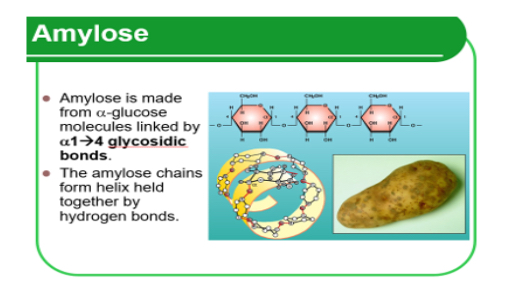
Amylose
• Polymer of a _1?.
• 1 → 4 glycosidic bonds which forms long chains. These coil into a _2?.
• Hydrogen bonds hold the helix structure and keep it _3?. This is good because it means that the plant can store a large amount of _4? molecules for the size.
1.Glucose. 2.Helix. 3.Compact. 4.glucose
Structure and function of starch
• 1_?- large amount of glucose stored
• 2_?- can't move out of cell
• 3_?- doesn’t affect water potential
• 4_?- easy to break down into glucose for respiration
1.Compact. 2.Too large. 3.Insoluble. 4.Has branches that creates ends
Glycogen is the energy storage polysaccharide of animals and _1?. Liver and muscles cells have a high concentration of glycogen, present as visible _2?, as the cellular respiration rate is high in these cells (due to animals being _3?).
1.Fungi. 2.granules. 3.Mobile
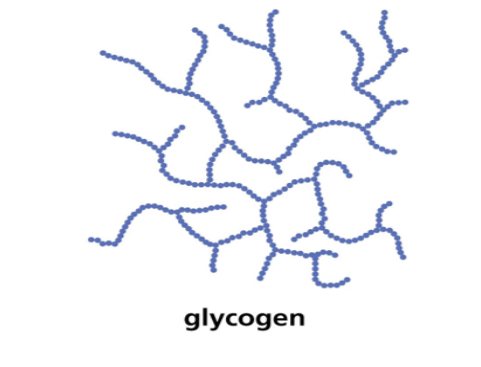
Structure and function of glycogen
• Polymer of a _1?.
• 1 → _2? glycosidic bonds
• BUT branched chains must be bonded as 1→ 6 _3? bonds
•Glycogen is branched(even more than amylopectin) making it more _4? which helps animals store more.
•The branching enables more _5? ends where glucose molecules can either be added or _6? allowing for condensation and hydrolysis reactions to occur more _7?- thus the storage or release of glucose can suit the demands of the cell.
•8_?-doesn’t affect water potential
1.glucose.
2. 4.
3.glycosidic.
4.Compact.
5. Free
6.removed
7.rapidly
8.insoluble
What is water potential?
The tendency of water to move from one area to another.(High water potential means the water has a low tendency to move out of the area. It indicates that the water molecules in that area have a higher energy state and are less bound or attracted to solutes or surfaces, making them less likely to move to another area.)
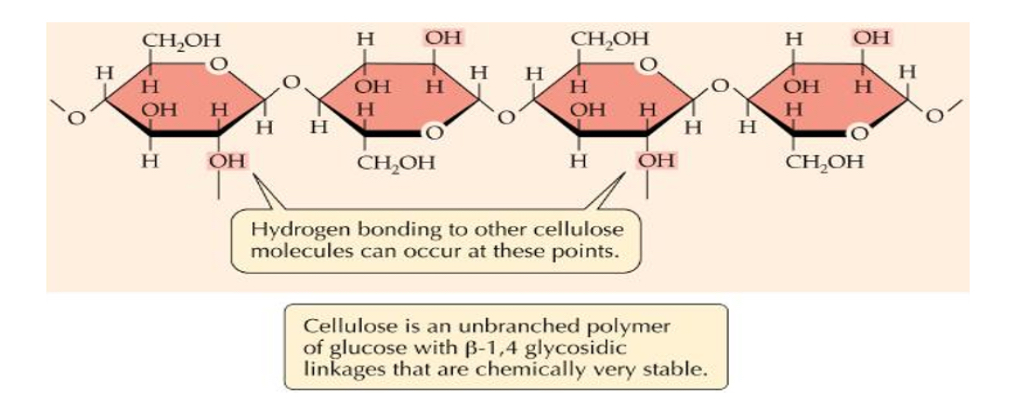
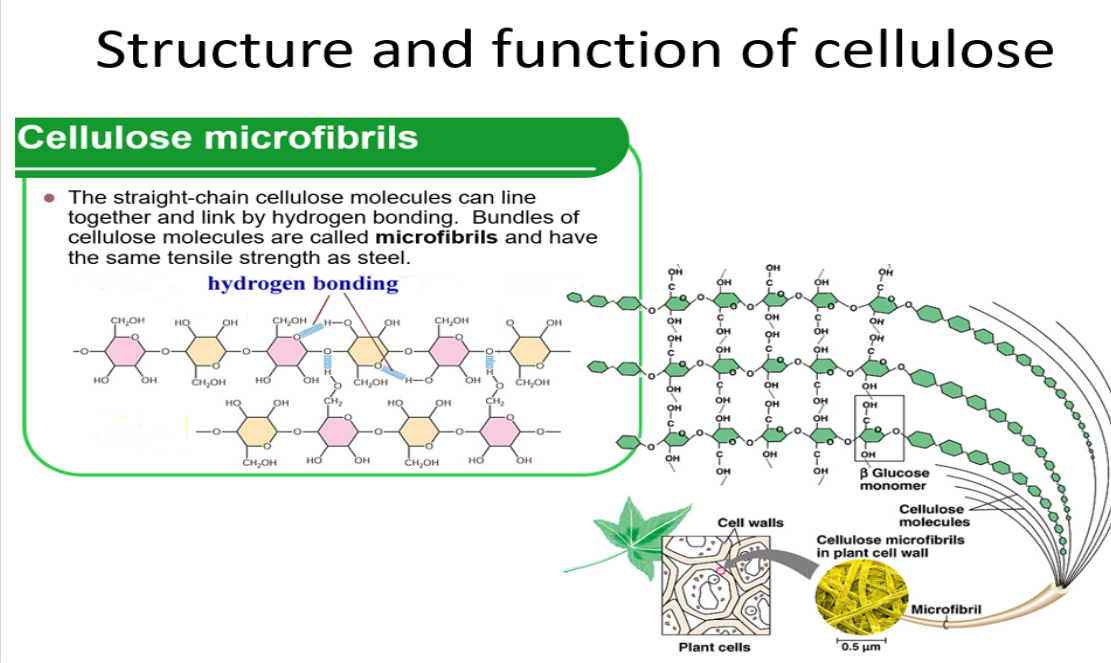
Structure and function of cellulose
• Polymer of _1?
• 1 → 4 glycosidic bonds • BUT every 2nd glucose molecule must be flipped _2? to allow bonding.
• Unbranched.
•Cellulose chains are linked together by _3? bonds to form strong _4? (microfibrils). These then form macrofibrils
• Lots of hydrogen bonds- high _5? strength of cellulose allows it to be stretched without breaking which makes it possible for cell walls to withstand _6? pressure(pressure exerted by the cell contents against the cell wall in plant and bacterial cells).
• Permeable to other molecules- Molecules such as water can move out of cell.
1.B-glucose
2.180 degrees
3.hydrogen
4.fibres
5.tensile
6.turgor
State 1 use of starch+glycogen and 3 uses of cellulose.
• Energy storage - starch (plants) and glycogen (animals)
• Structural support for plants - cellulose
• Main component of cell walls - cellulose
• Withstand turgor pressure in plants because of tensile strength -cellulose
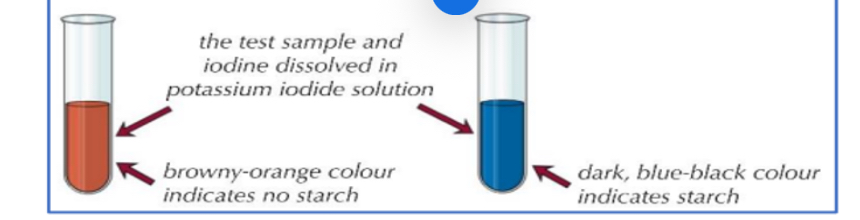
(Practical)-How do you test for starch?
1. To test for the presence of starch in a sample, add a few drops of orange/brown iodine in potassium iodide solution to the sample.
2. The iodine is in potassium iodide solution as iodine is insoluble in water.
3. If starch is present, iodide ions in the solution interact with the centre of starch molecules, producing a complex with a distinctive blue-black colour.
4. This test is useful in experiments for showing that starch in a sample has been digested