The Stratosphere & Ozone (O₃)
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Effect of UV radiation in humans
skin cancer
sunburn
premature aging of skin
Effect of UV radiation on other biodiversity
animal - skin cancer in animals
marine - plankton population threatened
plants - crop species impacted by lower growth
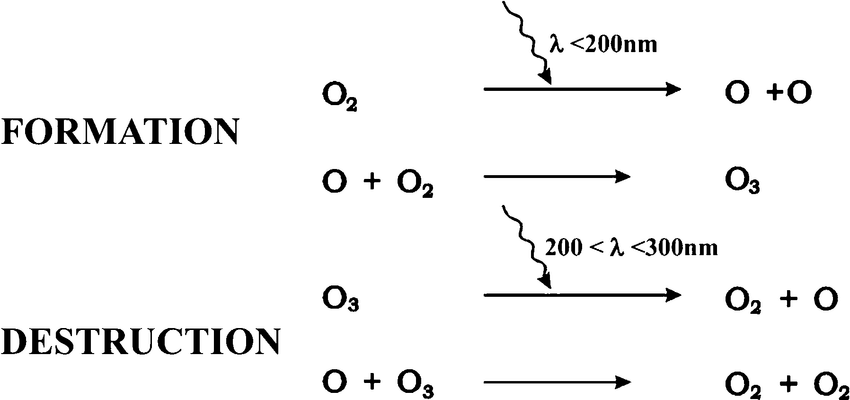
Ozone
is formed through a photochemical reaction, much less stable than oxygen and is very reactive
Ozone formation
creation: an oxygen molecule is split by a photon of light ( higher frequency UV light) into 2 oxygen atoms, the atomic oxygen produced quickly reacts with another oxygen molecule to reform ozone
destruction: if an oxygen and an ozone molecule meet,they recombine to form 2 oxygen molecules
Disadvantages of Chapman cycle
it predicts too much ozone as it doesn’t take into account for sinks, pathways that destroy ozone (oxide family)
Sources of oxide family
NOx - natural sources mainly also from fertilizer application
HOx - natural sources mainly also from agri application
ClOx & BrOx - nearly entirely due to human activity (CFC’s)
CFC’s (chlorofluorocarbons)
unreactive in the troposphere and moves unchanged to the stratosphere
Destruction of O3 by CFC’s
short wave UV light in stratosphere breaks down CFC’s and chlorine is released,
Cl + O3→ ClO + O2 (step 1)
ClO+O.→Cl+O2 (step 2)
O3+O.→2O2 (overall rxn)
Ozone Depleting Potential (ODP)
the ODP is the ratio of the impact on ozone of a chemical compared to the impact of a similar mass of CFC-11