Shapes of electron pairs
0.0(0)
0.0(0)
Card Sorting
1/6
Earn XP
Description and Tags
Electron pair = bond pair + lone pair
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
7 Terms
1
New cards
2 electron pairs
Linear
2
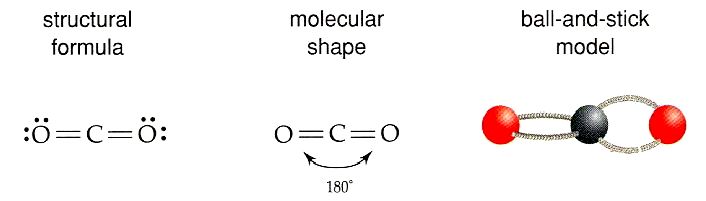
New cards
3 electron pairs
Trigonal planar
3
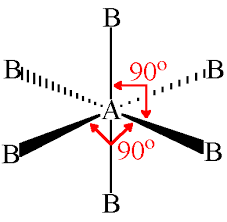
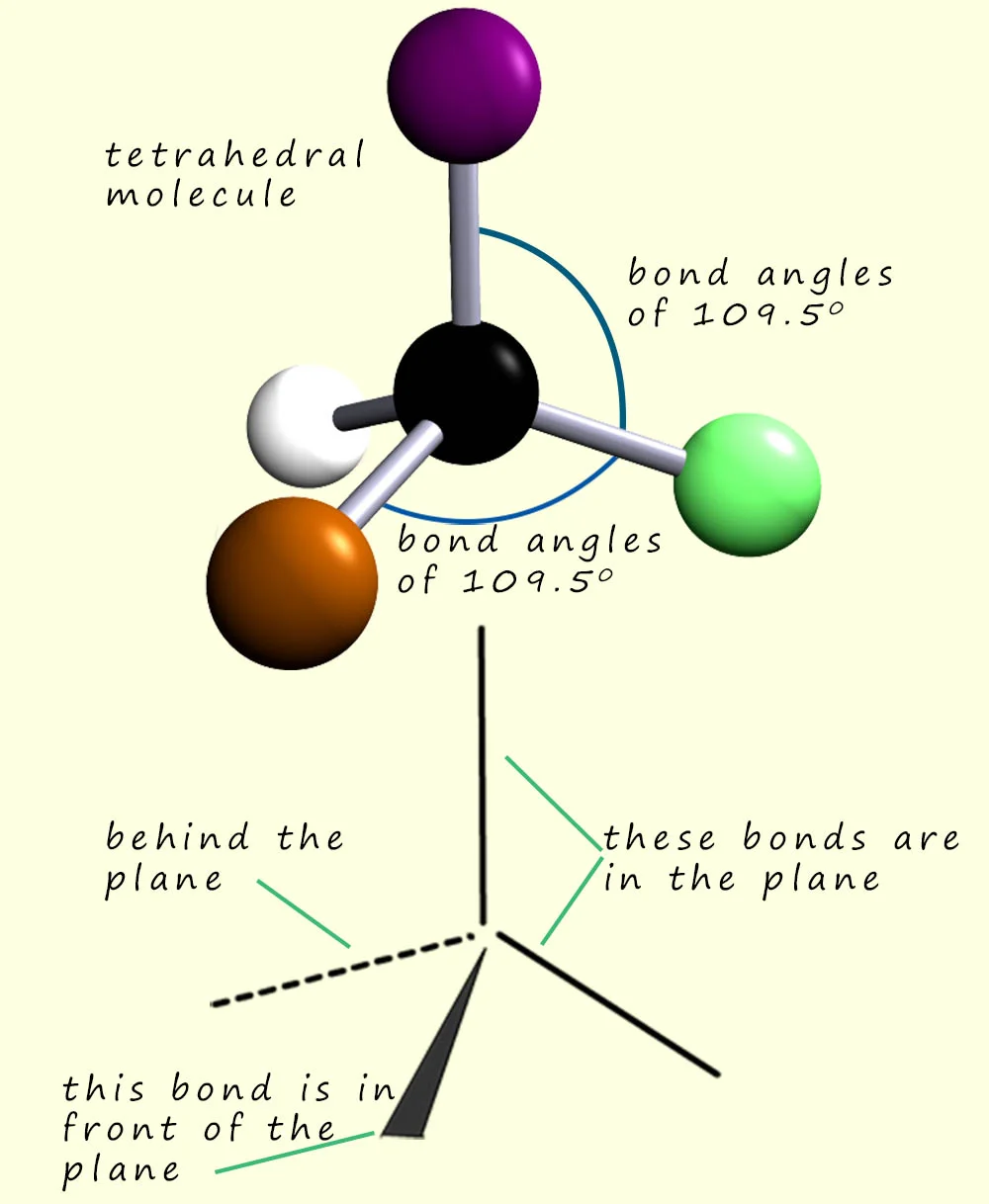
New cards
4 electron pairs
Tetrahedral
4
New cards
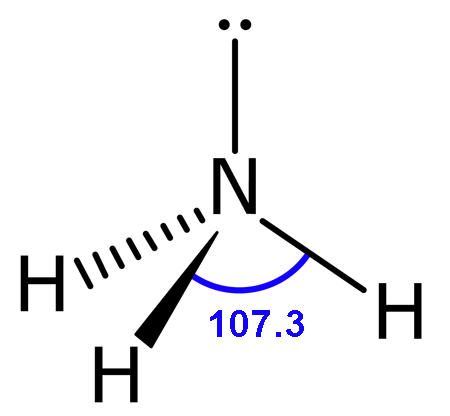
4 electron pairs (3 bond pairs + 1 lone pairs)
Trigonal pyramidal
5
New cards
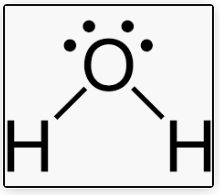
4 electron pairs (2 electron pairs + 2 lone pairs)
V-shaped
6
New cards
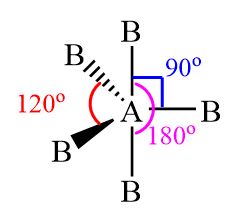
5 electron pairs
Trigonal bipyramidal
7
New cards
6 electron pairs
octahedral