Module 4 - Core Organic Chemistry
1/69
Earn XP
Description and Tags
OCR GCE A-Level Chemistry - Module 4 - Core Organic Chemistry
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms

'Dots' are used to represent species that are radicals within mechanisms.
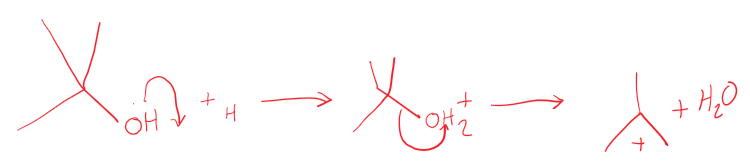
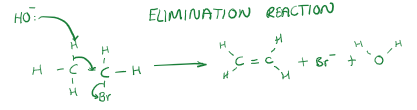
[4.1.1] What is an elimination reaction?
The reverse of an addition reaction, usually a smaller molecule is said to have been eliminated.
A **saturated hydrocarbon** containing **sigma bonds** (σ) which rotate freely.
Alkanes that are more highly branched have **lower boiling points** than alkanes with less branches. This is because branched chains reduce the amount of **London forces** that are able to form between alkane molecules.
\
* X₂ → X• + X•
\
In the first propagation step, a halogen radical reacts with a C-H bond in the alkane, forming a new radical and a **hydrogen halide** molecule.
\
* CH₄ + X• → •CH₃ + HX
\
In the second propagation step, each radical reacts with another halogen molecule, forming the **new organic product** and a **new halogen radical**.
\
* •CH₃ + X₂ → CH₃X + X•
\
The new halogen radical then reacts with another alkane to continue this process.
\
* X• + X• → X₂
\
* •CH₃ + •CH₃ → C₂H₆
\
* **Further substitution** can take place.
* Longer carbon chains lead to a **mixture of isomers**.
\
It locks the two carbon atoms in position and **prevents them from rotating**.
\
**Cis** → *2 identical groups are on the* ***same*** *side of C=C.*
**Trans** → *2 identical groups are on the* ***opposite*** *side of C=C.*
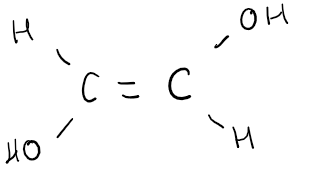
* Z is equivalent to Cis.
\
*This is only consistently correct when there is a* ***H on each carbon atom*** *of the C=C bond.*
\
The **priority group** of each carbon is the one with the largest atomic number, the positioning of the carbon priority groups is responsible for identification of the E/Z isomerism.
Addition reactions have an **atom economy of 100%**.
* Bromine **adds across the double bond**.
* The orange colour disappears.
[4.1.3] What is produced when an alkene reacts with a hydrogen halide?
They undergo an addition reaction to produce a haloalkane.
[4.1.3] What is the process of an alkene hydration reaction?
Alkenes react with steam, with a phosphoric acid catalyst - the steam adds across the double bond.
[4.1.3] What is produced in an alkene hydration reaction?
An alcohol.
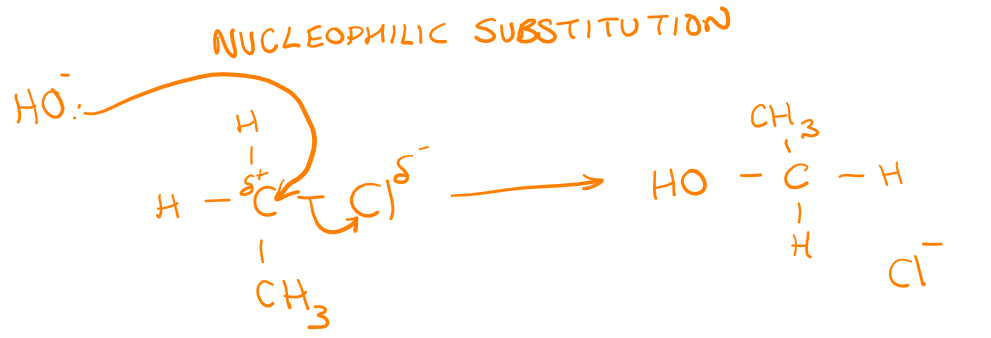
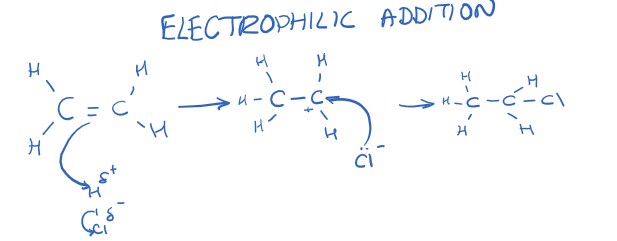
[4.1.3] What is an electrophilic addition reaction?
A reaction in which a substrate is attacked by an electrophile.
[4.1.3] What is the mechanism for electrophilic addition in alkenes?
[4.1.3] What is Markownikoff’s rule?
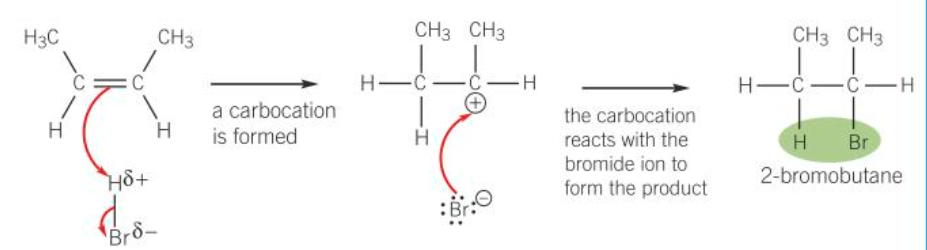
When a hydrogen halide reacts with an unsymmetrical alkene, the hydrogen of the hydrogen halide attaches itself to the carbon atom of the alkene with the greater number of hydrogen atoms and the smaller number of carbon atoms.
[4.1.3] How do we determine the major product of an alkene reaction?
A tertiary carbocation is more stable than a secondary and primary carbocation so will form the major product.
[4.1.3] What is an addition polymer?
The reaction in which many monomers containing at least one C=C double bond form long chains of polymers as the only product.
[4.1.3] What are the benefits of the combustion of waste polymers?
Polymers that are unrecyclable often have a high stored energy value; they are incinerated to produce heat, which generates steam to drive turbines which produce electricity.
[4.1.3] What are the benefits of feedstock recycling?
Feed-stock recycling breaks down polymers without separating them, forming simple gases that can then be used to manufacture pure, fresh polymers.
[4.1.3] What are biodegradable polymers?
Biodegradable polymers are broken down by microorganisms into water, carbon dioxide, and other biological compounds.
These polymers are usually made from starch or cellulose.
Compostable polymers degrade and leave no visible/toxic residue.
[4.1.3] What are photodegradable polymers?
These polymers contain bonds that are weakened by the absorption of light to begin the degradation process.
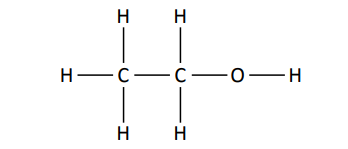
[4.2.1] What is an alcohol?
Alcohols contain the functional group ‘-OH’ where the oxygen is joined to a carbon by a single covalent bond.
Prefix - “Hydroxy”
Suffix - “-ol”
The related functional groups include “one”, “al”, “oic acid”, “thyl”, “anoate”.
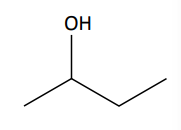
[4.2.1] How can alcohols be classified?
Alcohols are classified based on the number of carbons attached to the carbon that the -OH group is on.
Three Carbons → Tertiary Alcohol
Two Carbons → Secondary Alcohol
One or None → Primary Alcohol
e.g. 2-methylpropan-2-ol is a tertiary alcohol and ethanol is a primary alcohol.
[4.2.1] What is the oxidising mixture?
Acidified Potassium Dichromate
K₂Cr₂O₇/H₂SO₄
The oxidising mixture can be represented by [O].
[4.2.1] What are the first oxidation products of a primary alcohol?
An aldehyde and water.
[4.2.1] What is the further oxidation product of a primary alcohol?
A carboxylic acid.
[4.2.1] What is the first oxidation product of a secondary alcohol?
A ketone and water.
[4.2.1] How do we prevent further oxidations?
By using continuous distillation.
[4.2.1] How do we collect the final oxidation product?
Reflux.