chemical equilibrium chapter 17
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
reversible reaction
A reversible reaction is one in which the reactants react to form products that then react to give the reactants back i.e. the reaction is going in both directions and does not go to completion
chemical equilibrium
Chemical equilibrium is the dynamic state where the RATE OF the forward reaction is equal to the RATE OF the reverse reaction
dynamic state
reactants are continuously forming products and the products are continuously forming reactants
Does a reaction at equilibrium cease? Explain.
• No, a reaction at equilibrium has not ceased
• System is in a dynamic state – rate of forward reaction equals rate of reverse reaction
Looking at how equilibrium is reached for a reversible reaction
Take the reaction: A + B ←→C + D
a) Initially - Reactants present, no product present
b) Reaction begins - Products formed and start reacting, rate of reverse reaction increases
c) Equilibrium reached - Rate of forward reaction = rate of reverse reaction
Important points:
1) Once equilibrium is reached the concentrations of reactants and products remain constant as long as temperature remains constant
2) A reaction being at equilibrium does not mean that the concentration of products and reactants are equal
Where: [products] greater than [reactants] – equilibrium lies on the right
Where: [reactants] greater than [products] – equilibrium lies on the left
3) In a reaction at equilibrium, the reaction does not go to completion/does not cease and there will be some of all reactants and products involved present
le chateliers principle
if a stress is applied to a system then the system re-adjusts to relieve the stress applied
What effect does a catalyst have on equilibrium? Explain.
• None - A catalyst can bring a reaction to equilibrium more quickly but does not affect the state of equilibrium (Will not affect yields or colour changes)
• A catalyst speeds up the forward reaction and the reverse reaction equally - the dynamic equilibrium position is not changed.
temperature
Increasing temperature: Equilibrium will shift to decrease temperature - in the ENDOTHERMIC DIRECTION TO USE UP HEAT
Decreasing temperature: Equilibrium will shift to increase temperature - in the EXOTHERMIC DIRECTION TO PRODUCE HEAT
Exothermic reaction - produce heat - increase temperature - △H- (forward reaction)
Endothermic reaction - uses up heat - decreases temperature - △H+ (forward reaction)
pressure
Note : Very important
(1) Changing pressure will have no effect on equilibrium unless it is a gaseous reaction at i.e. where all reactants and products are gases
(2) Changing pressure will have no effect on equilibrium if there are equal numbers of molecules on each side of the equation
Increasing pressure: Equilibrium will shift to decrease pressure – in the direction that has LESS MOLECULES
Decreasing pressure: Equilibrium will shift to increase pressure - in the direction that has MORE MOLECULES
concentration
Increasing a substance’s concentration: Equilibrium will shift to decrease its concentration - in the direction that USES IT UP
Decreasing a substance’s concentration: Equilibrium will shift to increase its concentration – in the direction that produces MORE OF IT
ideal contiodns
high pressure low temp
Give two industrial applications of Le Chatelier’s principle: 1) Manufacture of ammonia
Known as the Haber process
N2 + 3H2 ←→ 2NH3
∆H = - 92.4 kJmol-1
Notice: An iron catalyst is used
According to Le Chatelier’s principle, what conditions should be used to maximise the yield of ammonia obtained?
- High pressures
- Low temperature
Why are these conditions not used in practice during the Haber process
- High pressures - uneconomical, danger of gas leaks and explosions
- Low temperatures – rate of reaction is too slow, takes too long to come to equilibrium
Note: Compromise pressure 200 atm is used
Compromise temperature 500°C is used
Give three uses of ammonia
- Fertilisers
- Explosives
- Cleaning product
Give two industrial applications of Le Chatelier’s principle: 2) Manufacture of sulfuric acid
• Known as the Contact process
• Three reactions involved - second reaction is a reversible reaction
Reaction 1:
S + O2 → 2SO2
Reaction 2:
2SO2 + O2 ←→ 2SO3
∆H = - 196 kJmol -1
Notice: A vanadium (V2O5) catalyst is used
Reaction 3:
SO3 + H2O → H2SO4
Why is a platinum catalyst not used?
Platinum is easily ‘poisoned’ by impurities in the reactants
According to Le Chatelier’s principle, what conditions should be used to maximise the yield of sulfuric acid obtained?
- High pressures
- Low temperatures
Why are these conditions not used in practice during the contact process?
- High pressures - uneconomical, SO3 will liquify at too high pressures
- Low temperatures – rate of reaction is too slow, takes too long to come to equilibrium
Note: Compromise pressure 1-2 atm is used (just above atmospheric pressure)
Compromise temperature 450°C is used
Give three uses of sulfuric acid
- Car batteries
- Detergents
- Paints
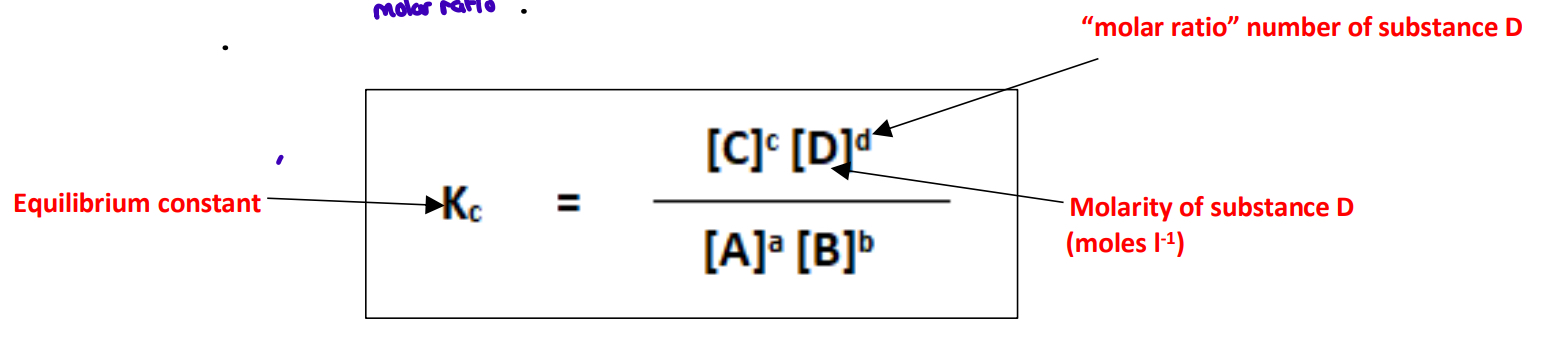
What is meant by the equilibrium constant (KC)?
The equilibrium constant shows the mathematical relationship between the concentration
of the reactants and the products for a reaction at equilibrium
The equilibrium constant
Important points:
1) The concentration of the products are on top, and concentration of reactants are on the bottom of the expression for Kc
2) This mathematical relationship is always the case provided temperature remains constant – only changing temperature changes the value of KC
Changing concentrations i.e. adding or removing a substance does not affect KC
Changing pressure does not affect Kc
DO NOT FORGET: TEMPERATURE IS THE ONLY FACTOR THAT AFFECTS KC
Kc is always constant at constant temperature
3) Kc greater than 1; concentration of products greater than reactants – equilibrium lies on right
Kc less than 1; concentration of reactants greater than products – equilibrium lies on left
Therefore: If equilibrium shifts to right and forward reaction is favoured, Kc increases
If equilibrium shifts to left and reverse reaction is favoured, Kc decreases
An increase in Kc means the forward reaction is being favoured (more products are being made)
A decrease in Kc means the reverse reaction is being favoured (more reactants are being made
Calculations involving KC
Type 1: Calculating KC from given moles/concentrations/mass/%
Steps:
1) Write out the balanced equation for the equilibrium reaction
2) Open up an ‘ICEE’ table and fill in all ‘known’ values
Notes - must be working in moles
‘Initial moles’ of the product (s) always = 0
(Unless initial value for product is given, in which case initial moles of reactant (s) = 0)
3) Use the molar ratio in the balanced equation to complete the ‘change in moles’ line
4) Complete the ‘ICEE’ table
Note - If the number of molecules is equal on both sides of the equation, the volumes will cancel in
the Kc expression
In this situation, volume is usually not given but even if it is it can be cancelled and
5) Fill in the molarities of reactants and products into the Kc equilibrium constant expressionand calculate KC
Calculations involving KC
Type 2: Calculating moles/concentration/mass/% from a given KC
Steps:
1) Write out the balanced equation for the equilibrium reaction
2) Open up an ‘ICEE’ table and fill in all ‘known’ values
Notes - must be working in moles
‘Initial moles’ of the product (s) always = 0
(Unless initial value for product is given, in which case initial moles of reactant (s) = 0)
3) Use ‘x’ to represent moles of reactants and products and use the molar ratio in the balanced equation to complete the ‘change in moles’ line in terms of ‘x’
4) Complete the ‘ICEE’ table in terms of ‘x’
Note - If the number of molecules is equal on both sides of the equation, the volumes will cancel in the Kc expression
In this situation, volume is usually not given but even if it is it can be cancelled
5) Fill in the molarities terms of ‘x’ of reactants and products into the Kc equilibrium constant expression and allow it to equal the known value for KC
6) Work as far as a quadratic equation and use to find ‘x’
(or if your maths is good, you might be able to use algebra)
Note: two values for x will be obtained using the quadratic formula – one will be impossible i.e. a negative value or too large
7) Use ‘x’ to find the quantity asked for in the question.