CHEM 14C - Functional Groups
1/33
Earn XP
Description and Tags
Riu, Winter 2026
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
alkane
only single bonds
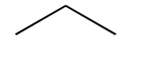
alkene
contains a C=C double bond
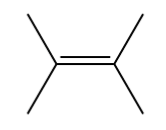
alkyne
contains a C≡C double bond
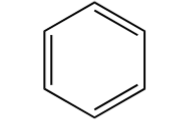
benzene ring
6-membered ring, 3 double bonds
hydro-carbons
composed of only H and C,
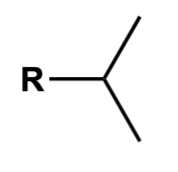
isopropyl
*R = non-hydrogen group
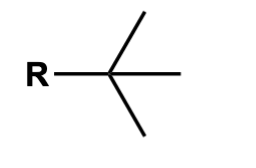
tert-butyl
*R = non-hydrogen group
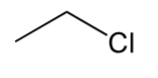
alkyl halide
Alkane where 1 hydrogen is replaced with halogen
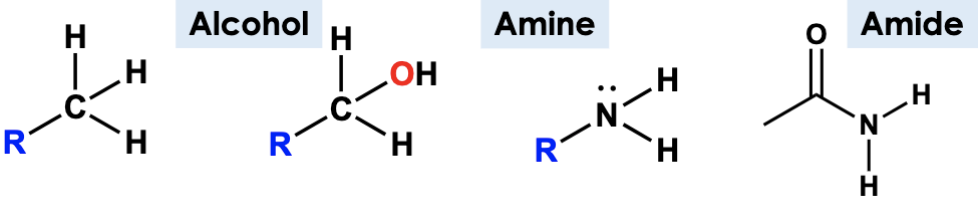
amine
N with 3 bonds and lone pair
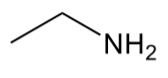
alcohol
have “C-O-H” group
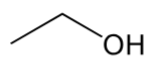
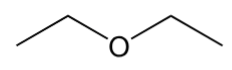
ether
have “C-O-C” group
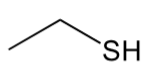
thiol
have a “C-S-H” group
sulfide
have a “C-S-C” group
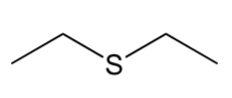
heteroatomic compounds
molecules containing atoms other than carbon and hydrogen, especially within ring structures (heterocycles) or as part of functional groups
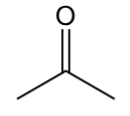
aldehyde
contain C=O with H and C bonded to C=O
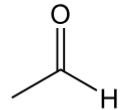
ketone
contain C=O with 2 C bonded to C=O
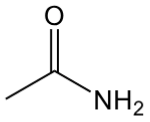
amide
contain C=O with N and C bonded to C=O
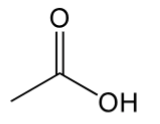
carboxylic acid
contain C=O with OH and C bonded to C=O carbon
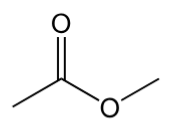
ester
contain C=O with ‘O-C’ and C bonded to C=O carbon
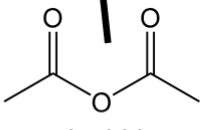
anhydride
contain O atom that bridges two C=O’s
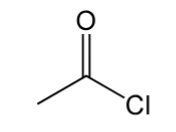
acid chloride
contain C=O with Cl and C bonded to C=O
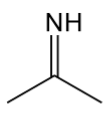
imine
contains a C=N (N bonded to H or C, C bonded to H or C)
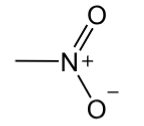
nitro
contains a -NO2
nitrile
contains a C≡N
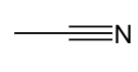
carbonyl compounds
organic molecules featuring a carbon atom double-bonded to an oxygen atom (C=O), forming a polar, electrophilic group central to many classes
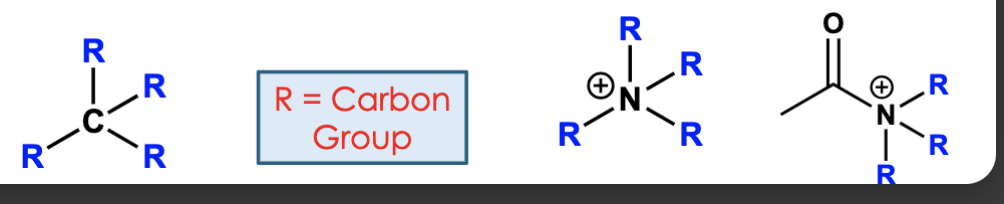
primary degree designation (1°)
only connected to one carbon group
secondary degree designation (2°)
connected to two carbon groups
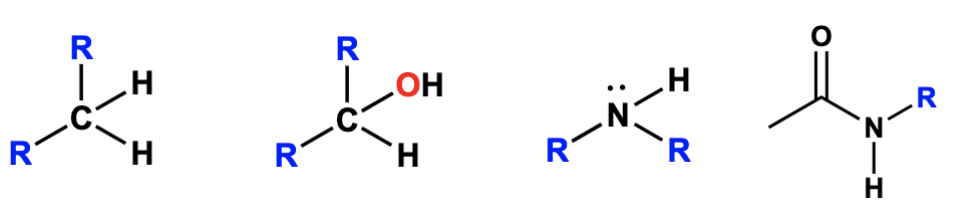
tertiary degree designation (3°)
connected to one carbon group
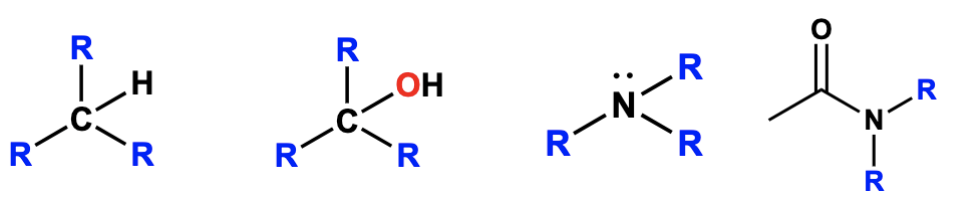
quatenary degree designation (4°)
connected to four carbon groups
meythl
ethyl
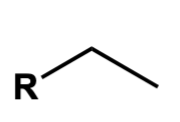
propyl
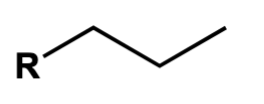
butyl
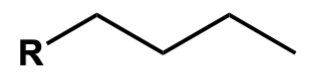
Mice, Eat, Peanut, Butter, Perhaps, Hamburgers
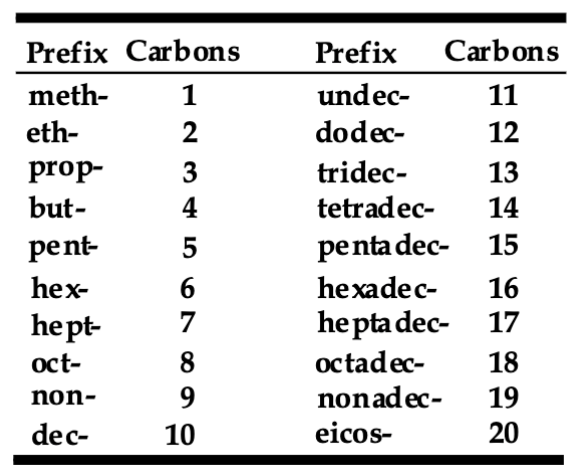
Meth, Eth, Prop, Butyl, Pent, Hex