Midterms: Lesson 3 - Subatomic Particles
1/16
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Dalton’s Atomic Theory
A theory of chemical combination, first stated by John Dalton in 1803.
It involves the following postulates:
Elements consist of indivisible small particles (atoms).
All atoms of the same element are identical; different elements have different types of atom.
Atoms can neither be created nor destroyed.
One important change, however, is that atoms are now known to be divisible.
They can be broken down into even smaller, more fundamental particles, called subatomic particles.
Electrons
Protons
Neutrons
What are three kinds of subatomic particles?
Electrons
In 1897, the English physicist J. J. Thomson (1856–1940) discovered this type of subatomic particle.
They are negatively charged subatomic particles.
Cathode Ray Experiment
Thomson performed experiments that involved passing electric current through gases at low pressure.
He sealed the gases in glass tubes fitted at both ends with metal disks called electrodes.
The electrodes were connected to a source of electricity.
One electrode, the anode became positively charged, while the other electrode, the cathode, became negatively charged.
The result was a glowing beam, or cathode ray, that traveled from the cathode to the anode.
Thomson found that a cathode ray is deflected by electrically charged metal plates.
A positively charged plate attracts the cathode ray, while a negatively charged plate repels it.
Corpuscles / Electrons
Thompson knew that opposite charges attract and like charges repel, so he hypothesized that a cathode ray is a stream of tiny negatively charged particles moving at high speed.
Thompson called these particles ___. Later thy were named ___.
Thompson concluded that ____ are a component of the atoms of all elements.
1/1840
An electron has one unit of negative charge, and its mass is ___ the mass of a hydrogen atom.
Plum-Pudding Model
Gold Foil Experiment
Rutherford Atomic Model
What were the models used to describe the structure of the nuclear atom? (Atomic Nucleus)
How can you describe the structure of the nuclear atom?
In the nuclear atom, the protons and neutrons are located in the nucleus. The electrons are distributed around the nucleus and occupy almost all the volume of the atom.
Plum-Pudding Model
J. J. Thompson— thought it was likely that the electrons were evenly distributed throughout an atom filled uniformly with positively charged material.
Electrons were stuck into a lump of positive charge, similar to raisins stuck in dough.
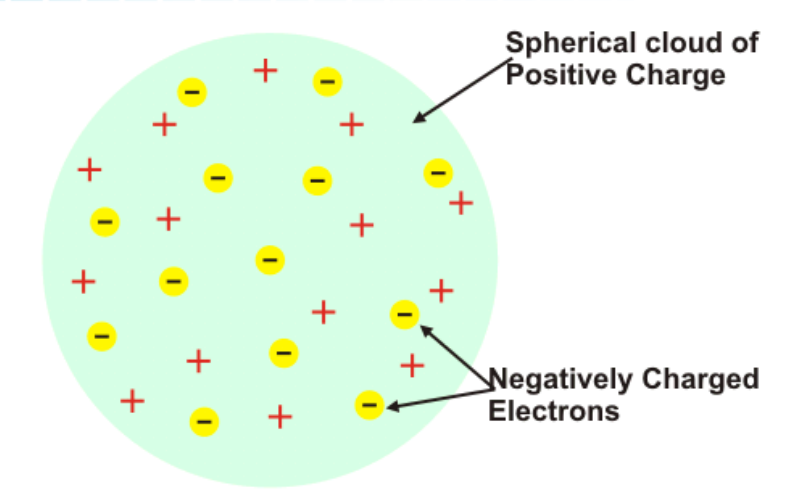
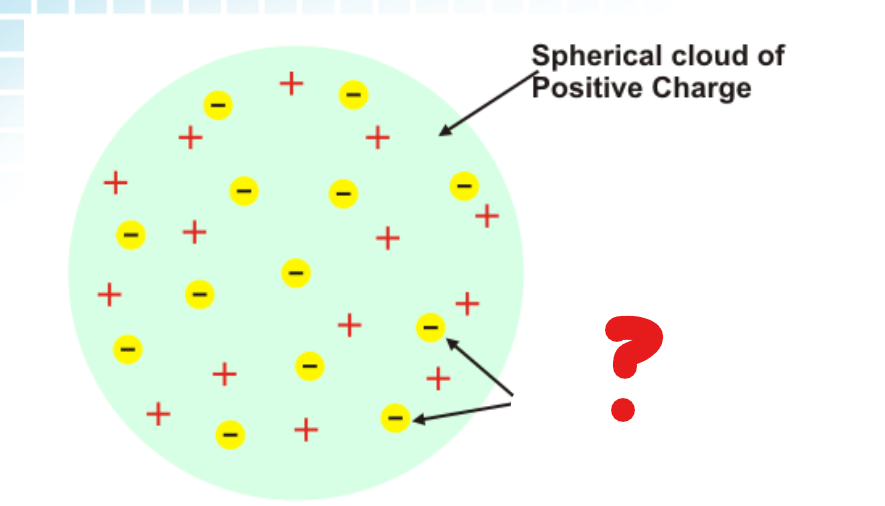
Spherical Cloud of Positive Charge
What part of the Plum-Pudding model is this?
Negatively Charged Electrons
What part of the Plum-Pudding model is this?
Ernest Rutherford
Born in New Zealand, This person was awarded the Nobel Prize for Chemistry in 1908. His portrait appears on the New Zealand $100 bill.
Gold-Foil Experiment
In 1911, Rutherford and his co-workers wanted to test the existing plum-pudding model of atomic structure.
They devised the gold-foil experiment.
Their test used alpha particles, which are helium atoms that have lost their two electrons and have a double positive charge because of the two remaining protons.
In the experiment, a narrow beam of alpha particles was directed at a very thin sheet of gold.
According to the prevailing theory, the alpha particles should have passed easily through the gold, with only a slight deflection due to the positive charge thought to be spread out in the gold atoms.
The results were that most alpha particles went straight through, or were slightly deflected.
What was surprising is that a small fraction of the alpha particles bounced off the gold foil at very large angles.
Some even bounced straight back toward the source.
Cathode Ray
A stream of electrons produced at the negative electrode (cathode) of a tube containing a gas at low pressure/
Proton
A positively charged subatomic particle found in the nucleus of an atom
Neutron
A subatomic particle with no charge and a mass of 1 amu; found in the nucleus of an atom
Nucleus
The tiny, dense central portion of an atom, composed of protons and neutrons