CP - The Chemistry of Life
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
atom
the basic unit of a chemical element
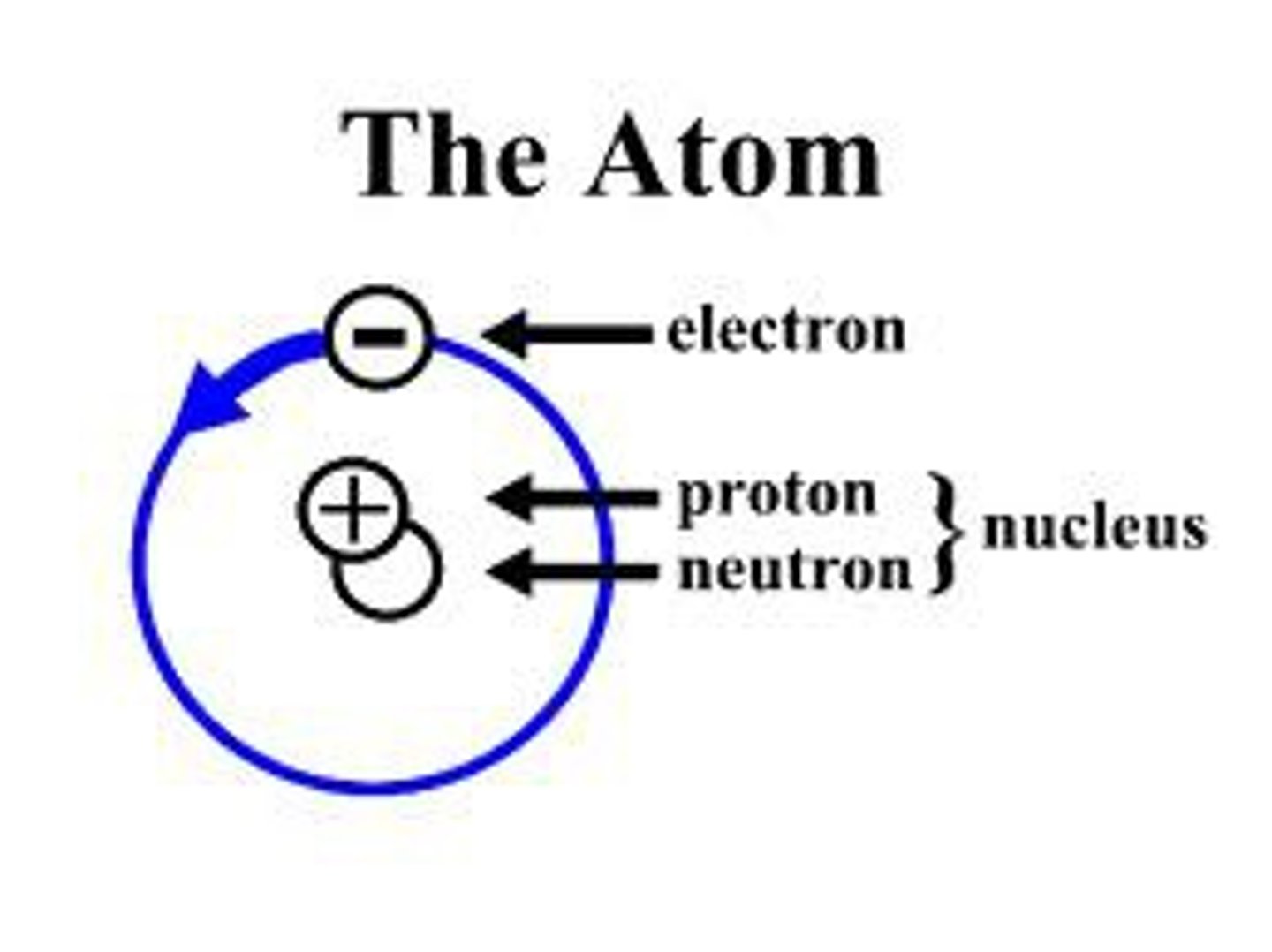
proton
a subatomic particle that has a positive charge and that is found in the nucleus of an atom
# of protons = atomic number

electron
a subatomic particle that has a negative charge and found outside the nucleus
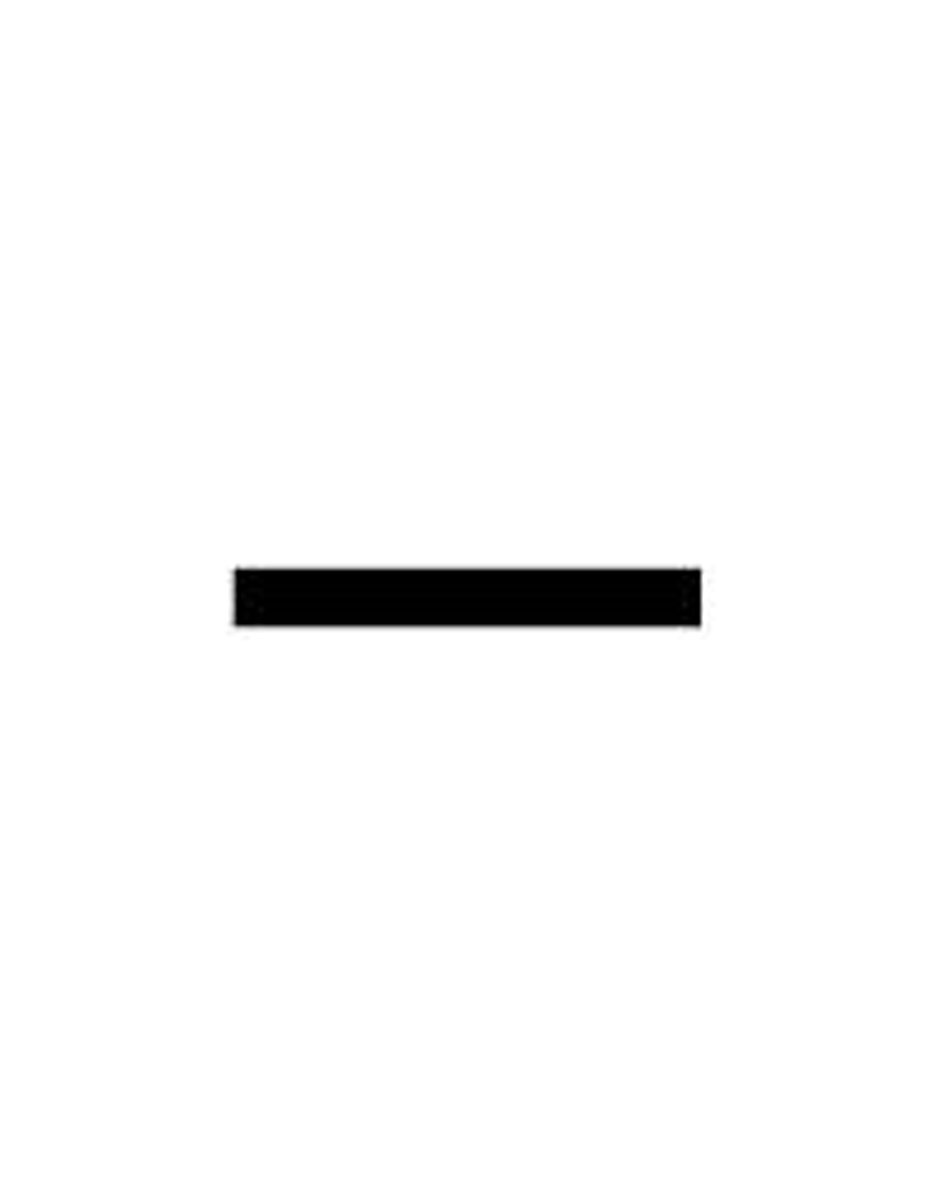
neutron
a subatomic particle that has no charge and that is found in the nucleus of an atom
# of neutrons + # of protons = atomic mass
neutral
in an atom, the positive charges of the protons are negated by the negative charges of the electrons; protons and electrons will be equal in number
element
a pure substance made of only one kind of atom
nucleus
the dense center of an atom made up of protons and (except in the case of a hydrogen atom) neutrons
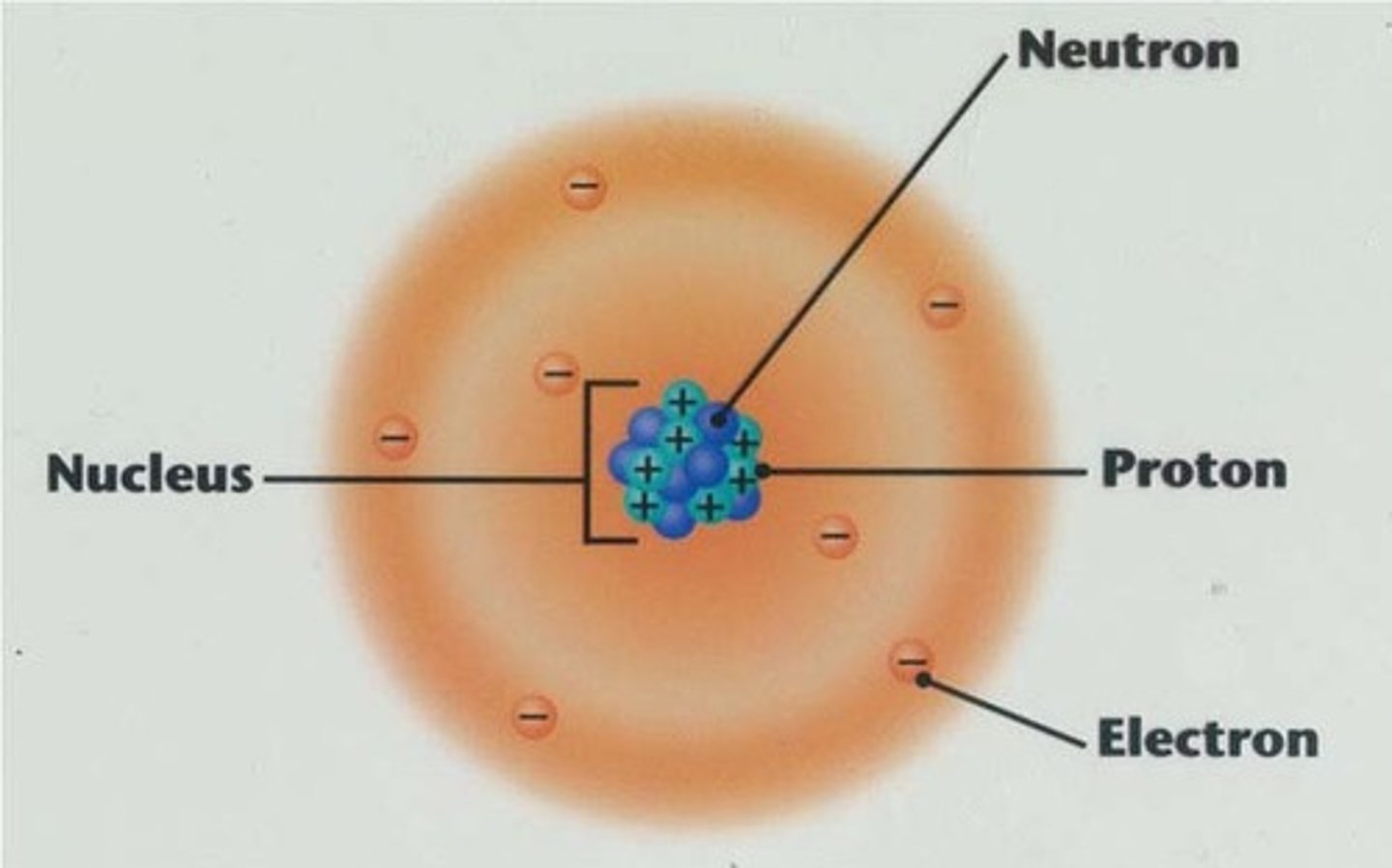
electron cloud
area around the nucleus of an atom where the atom's electrons are most likely to be found

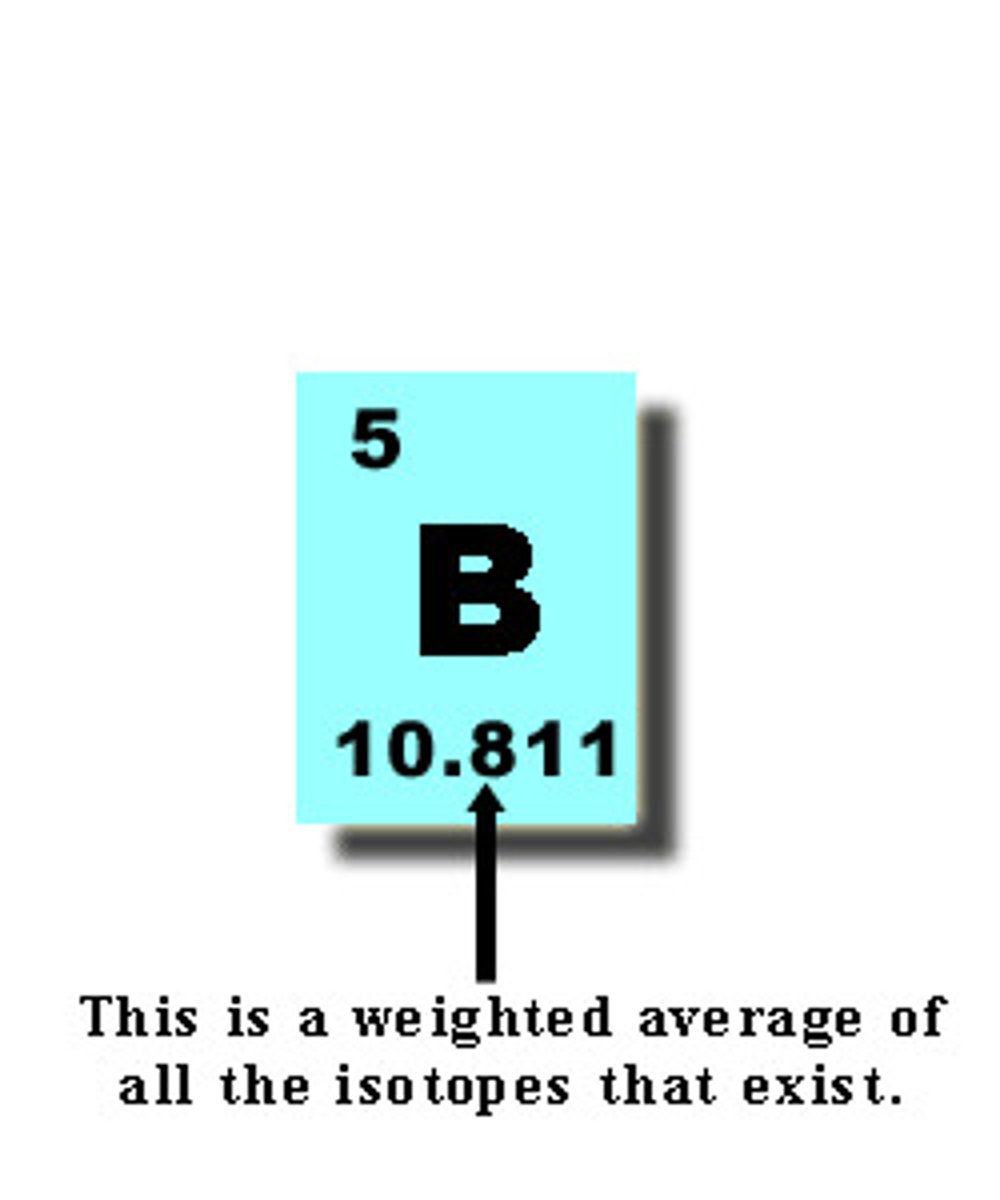
atomic mass
the weighted average of the masses of the isotopes of an element; protons + neutrons
atomic number
the number of protons in the nucleus of an atom

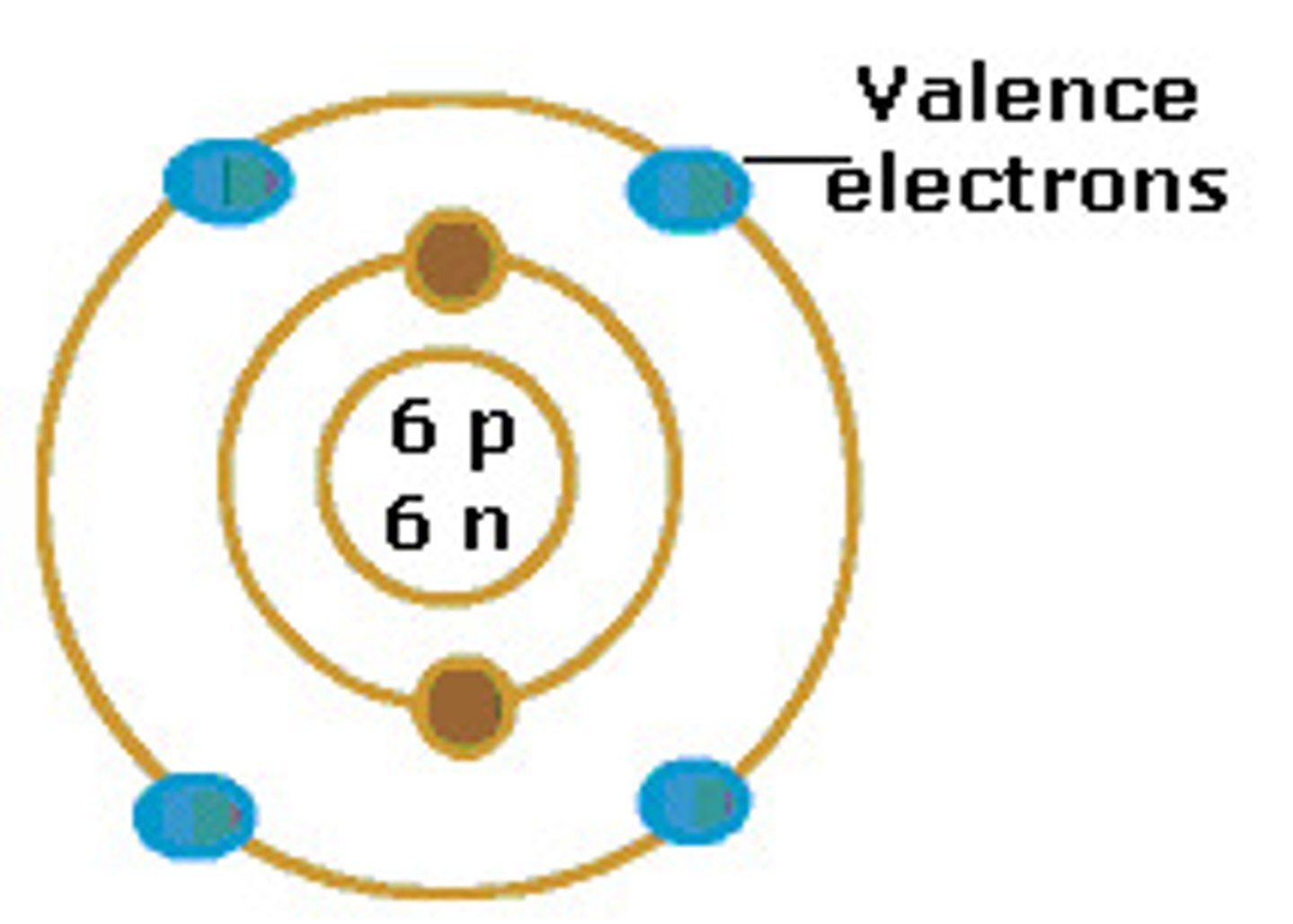
valence electrons
the electrons in the outermost shell (main energy level) of an atom; these are the electrons involved in forming bonds
ionic bond
a chemical bond resulting from the attraction between oppositely charged ions; typically when a metal and a nonmetal bond
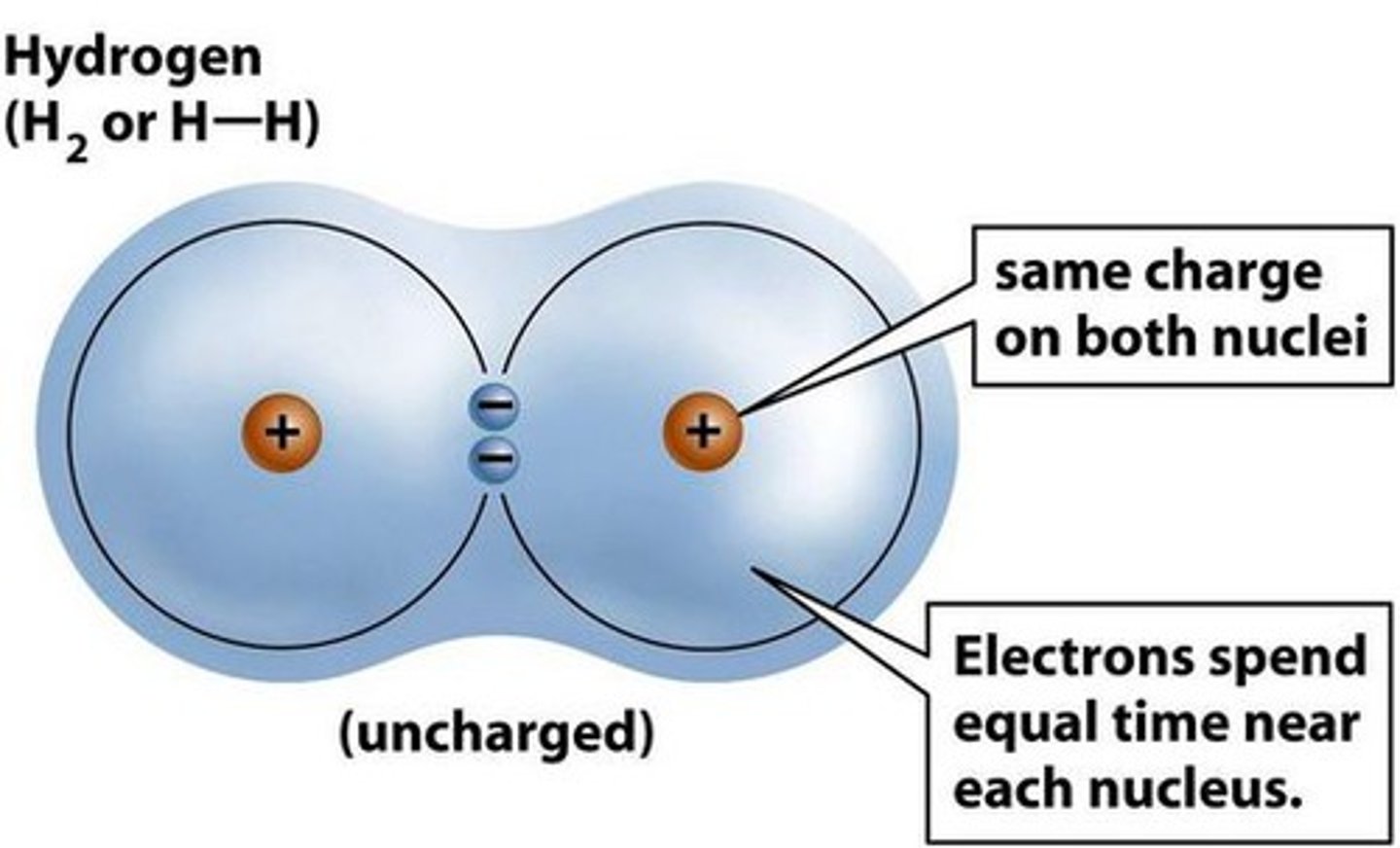
covalent bond
a chemical bond that involves sharing a pair of electrons between atoms in a molecule; typically when two nonmetals bond
hydrogen bond
attraction between a slightly positive hydrogen atom and a slightly negative atom; very weak bonds that exist between the bases in DNA
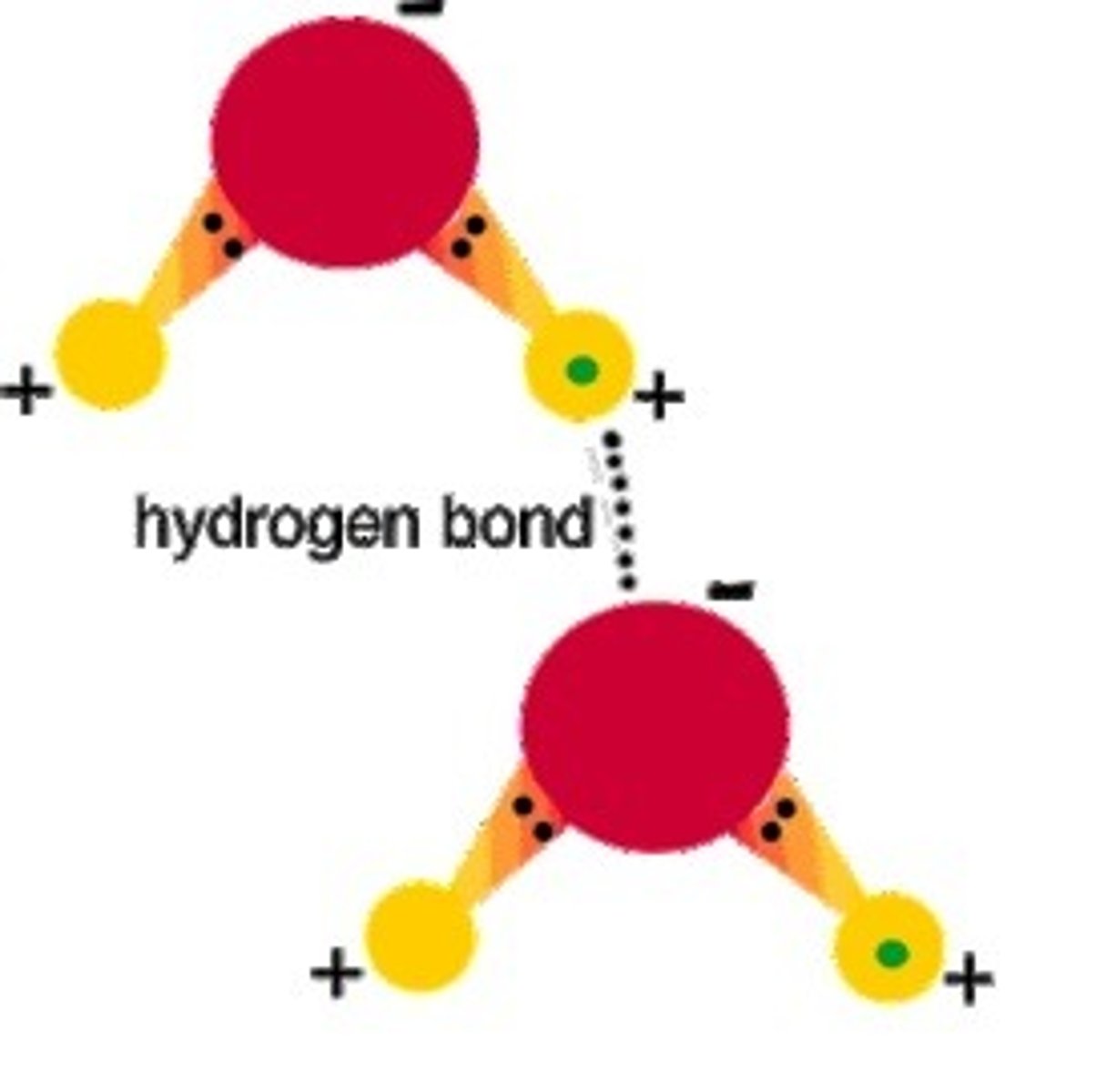
cohesion
an attraction between molecules of the same substance

adhesion
attraction between molecules of different substances

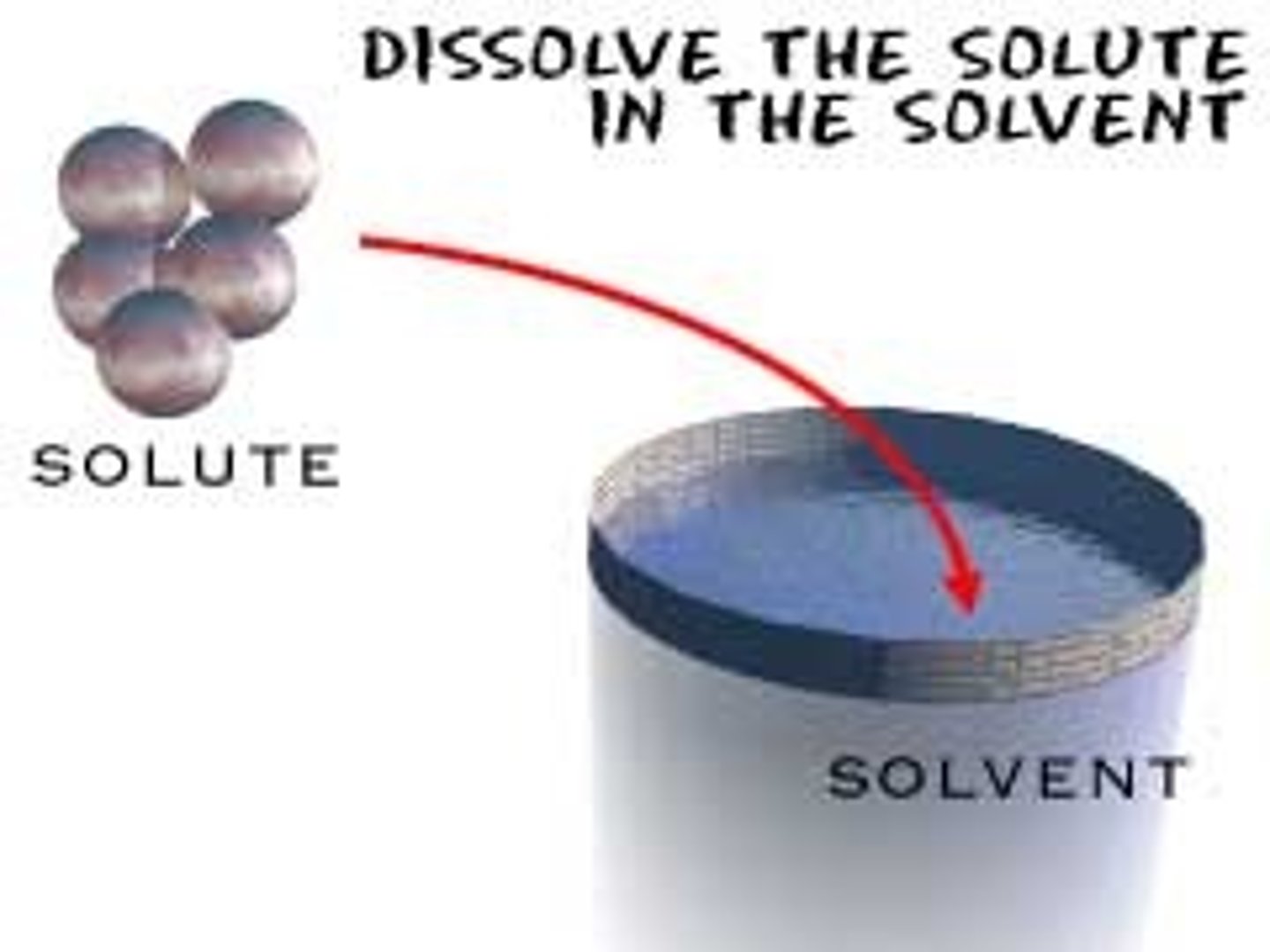
solute
substance being dissolved

solvent
the substance in which the solute dissolves
acid
a substance that increases the hydrogen ion (H+) concentration of a solution; has a pH range of 0-7
base (alkaline)
compound that produces hydroxide ions (OH-) in solution; has a pH range of 7-14