Pneumonia
1/85
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
86 Terms
2005 HAP/VAP/HCAP Guidelines
Is it a late onset (patient is in hospital for >5 days)?
or
Does the patient have a risk factor multiple drug resistance?
No- Use limited spectrum antibiotic therapy (less aggressive antibiotics)
Yes- Use broad spectrum antibiotic therapy (more aggressive)
Risk Factors for Multiple Drug Resistance
Antibiotics used in the last 90 days
>5 days hospitalized
High frequency of antibiotic resistance in the community or hospital
Any of the following HCAP risk factors:
Stayed in a hospital for 2 or more days in the last 90 days
Lives in a nursing home or long-term care facility
Gets home IV antibiotics
On chronic dialysis within the last 30 days
Receives home wound care
Lives with someone who has drug-resistant infections
Has a weakened immune system
What happened after the 2005 HCAP guidelines came out?
Studies looked into the guidelines and found problems:
No clear improvement in patient outcomes
The criteria didn’t do a good job identifying patients with drug-resistant bacteria
Resulted in 15 years of antibiotic overprescribing
Doctors still sometimes use the old HCAP definition to justify giving strong antibiotics, even though it’s no longer recommended
Pathophysiology of pneumonia
Microbe invades the airway
Bacteria gets into the lungs through:
Inhalation
Aspiration (accidentally breathing in food, saliva, or vomit)
Bloodstream spread from another infected part of the body
Body’s defence are overcome
Normally, your lungs have defense systems like:
Mucociliary clearance – tiny hairs and mucus that trap and remove germs
Macrophages – immune cells that “eat” invaders
But in pneumonia:
These defenses are damaged or not working well
Bacteria slip through and start growing
Bacteria grow and cause inflammation
Bacteria reach the alveoli (tiny air sacs in the lungs)
The immune system reacts:
Macrophages call in neutrophils (another type of immune cell)
These cells release cytokines (chemical signals) to fight infection, but this also causes inflammation
Signs / Symptoms of pneumonia
New or worsening sputum (saliva) production
New or worsening cough
Increased respiratory rate
Pleuritic chest pain
Sharp chest pain that gets worse when you take a deep breath or cough
Leukocytosis / Leukopenia
Fever / Hypothermia
Auscultatory changes
Like crackles or rales when doctor listens to your chest
Decrease in oxygenation
Diagnosis of Pneumonia
Chest X-ray is the main tool to use
What do doctors look for in the x-ray:
Infiltrate or consolidation
These are cloudy areas that suggest infection or fluid in the lungs
May indicate pneumonia, but…
The X-ray can’t tell if the pneumonia is caused by bacteria or viruses
What if the X-ray looks normal, but the patient has symptoms?
Wait 24–48 hours and then repeat the X-ray
Sometimes early pneumonia doesn’t show up right away on the image
Sputum Analysis
It’s when doctors test the mucus (sputum) you cough up to see which bacteria might be causing your pneumonia
But there’s a challenge…
40–60% of people with pneumonia can’t produce sputum at all
Of the ones who can, many samples are poor quality and not helpful
When should cultures definitely be done?
If the pneumonia is severe (e.g., ICU patients)
If the patient is being treated for MRSA or Pseudomonas aeruginosa
These are serious drug-resistant bacteria, so it’s important to know for sure
What makes a “good” sputum sample?
Lots of PMNs (immune cells which help fight infection)
Few epithelial cells from the mouth
Specifically:
More than 25 PMNs
Less than 10 epithelial cells
This tells us the sample likely came from the lungs, not just spit
Procalcitonin
A substance normally made in the thyroid (by C-cells)
In healthy people, it's turned into calcitonin (a hormone that helps regulate calcium)
What happens during a bacterial infection?
Other parts of the body, like fat cells (adipocytes), start making lots of procalcitonin (PCT) in response to bacterial signals (like LPS, IL-1β, TNF-α)
These cells can’t convert PCT to calcitonin, so PCT builds up in the blood
High PCT = likely bacterial infection
What about viral infections?
Interferon-γ (IFN-γ), which is high during viral infections, blocks PCT production
So PCT levels usually stay low in viral infections
Why is this useful?
Doctors can measure PCT levels in the blood to help decide:
Is the infection bacterial (high PCT)?
Or viral (low PCT)?
It’s especially studied in:
CAP (community-acquired pneumonia)
Sepsis
PCT levels usually peak within 24 hours after a bacterial infection starts
Sometimes it rises a little with viral infections too, but then it quickly drops
The most common PCT level used to suggest bacterial pneumonia is…..
> 0.25 ng/mL
Pneumonia Testing Algorithm
A patient comes in with signs of an LRTI (like cough, fever, shortness of breath)
Do a clinical assessment (look at symptoms, vitals, exam, etc.)
Perform a PCT test
a. If PCT: < 0.1 ng/mL
Very low likelihood of bacterial infection
Antibiotic therapy strongly discouraged
b. PCT: 0.1 - 0.25 ng/mL
Low likelihood of bacterial infection
Antibiotic therapy discouraged
c. PCT: 0.25-0.5 ng/mL
Higher chance of bacterial infection
Antibiotic therapy encouraged
d. PCT: >0.5 ng/mL
Strong evidence of bacterial infection
Antibiotic therapy strongly encouraged
Things to Consider with PCT
If the patient is unstable, high-risk, or has severe symptoms, antibiotics might still be started, even if the PCT is low
Re-evaluation is recommended regularly to avoid unnecessary prolonged antibiotic use
Repeat Testing:
Low-risk: Repeat PCT in 1–2 days
Moderate-risk: Repeat in 6–12 hours
High-risk / On antibiotics: Recheck every 2–3 days, and stop antibiotics early if PCT drops
2019 Guideline Recommendations for PCT
Do not use PCT to decide whether to start antibiotics if:
The patient already has pneumonia confirmed on a chest X-ray
And has symptoms (like cough, fever, or low oxygen)
So, even if the PCT is low, if the X-ray and clinical signs point to pneumonia → still treat
Very low (i.e. ≤ 0.1 µg/mL) levels typically indicate lack of bacterial infection
Higher PCT levels may mean bacterial infection, but:
There’s no official cutoff that clearly separates viral from bacterial
In cases where the infection is both viral and bacterial, the PCT might still be low, making it less reliable
Using PCT to track progress (serial testing) doesn’t seem to help much in shortening how long patients are on antibiotics
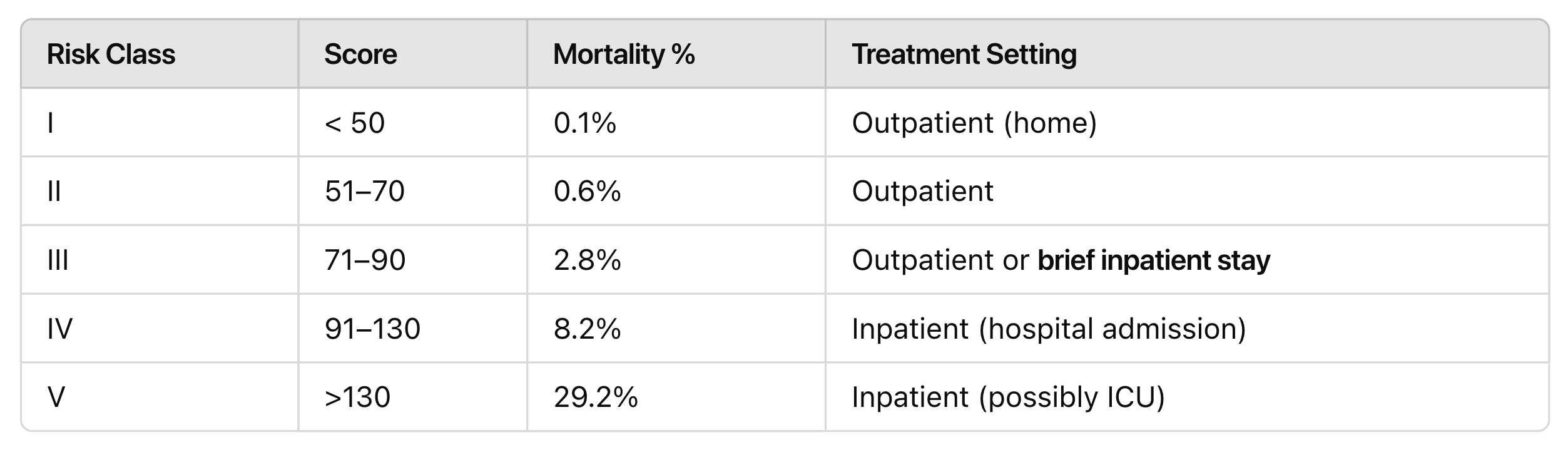
Pneumonia Severity Index Scoring
CURB-65
1 point each:
C – Confusion
U – Uremia (BUN > 20 mg/dL)
R – Respiratory rate > 30 bpm
B – Blood pressure < 90/ 60
Age 65 – Age ≥ 65 years
Score | Treatment Setting |
0-1 | Outpatient |
2 | Inpatient- floor |
≥ 3 | Inpatient- ICU |
Why do guideline recommend PSI over CURB65?
Identifying low-risk patients → more patients can safely be treated at home
Predicting mortality → more accurate in telling who is truly at risk
Validated in studies → proven useful in multiple clinical trials
But always combine with clinical judgment — because scores don't know everything
Examples:
A patient may still need admission if:
They have medical issues (e.g., unstable heart disease)
They have psychosocial issues (e.g., live alone, can’t care for themselves)
PSI might underestimate risk in younger patients since younger age lowers the score, even if they’re really sick
You must also think about their baseline health (e.g., a chronically ill patient might appear “low risk” on paper)
Determining ICU Admission for Pneumonia
IDSA (Infectious Diseases Society of America) severity criteria helps to decide
It works alongside clinical judgment, it doesn’t replace it!
ICU admission is recommended if:
The patient meets 1 major criterion
ORMeets 3 or more minor criteria
Other tools like SMART-COP exist, but:
They may need extra lab work
They don’t seem to work better than IDSA’s criteria
IDSA is more practical and validated
IDSA Major Criteria
Need only 1
IDSA Minor Criteria
Needs 3 or more to be allowed in the ICU
Most common Bacterias that Cause pneumonia
Strep pneumonia
Haemophilus influenzae
Mycoplasma pneumoniae
Clamydophilia pneumoniae
Legionella pneumophilia
S. aureus (rare, risk factors for infection)
Community-Acquired Pneumonia (CAP)
Most of the time… we don’t know the exact cause!
In up to 62% of cases, no pathogen is identified, even after testing
24% are viral pathogens
14% are bacterial pathogens
About 3% of cases have both bacteria and virus at the same time
Risk Factors for MRSA and Pseudomonas aeruginosa in CAP
It’s rare for CAP to be caused by MRSA or Pseudomonas
These are resistant and serious bacteria, but not common in most community cases
Most consistent risk factors:
Previous infection with MRSA or Pseudomonas
Especially if it was a lung infection (like pneumonia or bronchitis)
Recent hospitalization WITH IV antibiotics
If the patient was in the hospital and received IV antibiotics within the past 90 days
MRSA in the community
Some MRSA strains produce a toxin called Panton-Valentine Leukocidin (PVL)
This toxin kills white blood cells and damages lung tissue
The result is cavitary pneumonia , areas of the lung break down and form holes or cavities
Still very uncommon overall
All Staphylococcus aureus infections make up only ~1% of all community-acquired pneumonia (CAP)
First Line: Outpatient, otherwise healthy
Amoxicillin
OR
Doxycycline
Alternative Line: Outpatient, otherwise healthy
if local resistance to S. pneumoniae is <25%
Azithromycin
OR
Clarithromycin
Outpatient WITH comorbidities examples
chronic heart/lung/liver/renal disease
diabetes
asplenia
malignancy
alcoholism
First Line: Outpatient WITH comorbidities
Amoxicillin/clavulanate
OR
Cefpodoxime
OR
Cefuroxime
PLUS
Azithromycin OR doxycycline
Alternative Line: Outpatient WITH comorbidities
Levofloxacin
OR
Moxifloxacin
First Line: Inpatient, non-ICU, no MRSA/P. aeruginosa risk factors
IV β-lactam PLUS azithromycin
IV Beta-Lactams
Ceftriaxone
Ceftaroline
Cefotaxime
Ampicillin-sulbactam
Alternative Line: Inpatient, non-ICU, no MRSA/P. aeruginosa risk factors
Levofloxacin
OR
Moxifloxacin
ORIV β-lactam + doxycycline
First Line: Inpatient, non-ICU, recent IV antibiotics
IV β-lactam PLUS azithromycin
First Line: Inpatient, non-ICU, Recent MRSA infection
IV β-lactam PLUS azithromycin + MRSA coverage
First Line: Inpatient, non-ICU: Recent P. aeruginosa infection
Antipseudomonal IV β-lactam
First Line: Inpatient, ICU: no MRSA/P. aeruginosa risk factors
IV β-lactam PLUS azithromycin
Alternative Line: Inpatient, ICU: no MRSA/P. aeruginosa risk factors
IV β-lactam
PLUS
Levofloxacin
OR
Moxifloxacin
First Line: Inpatient, ICU: with risk factors
Vancomycin
OR
Linezolid
PLUS
Antipseudomonal β-lactam
What if cultures come back negative with MRSA/Pseudomonas?
If cultures are negative at 48 hours, you can stop MRSA or Pseudomonas drugs safely
If the MRSA nasal PCR is negative, you can stop vancomycin or linezolid
For MRSA - you can stop if either of those are happening
MRSA Nasal PCR
Quick nose swab test that checks if MRSA is living in your nose (colonization)
This doesn’t mean you’re sick, just that MRSA is hanging out there
If the nasal PCR is negative, it means the patient probably does not have MRSA pneumonia.
So: You can safely stop MRSA drugs
A positive just means the person has MRSA in their nose,
but doesn’t confirm they have MRSA in the lungsSo: You can’t make decisions based on a positive alone, you still need to look at cultures and symptoms
Pneumococcal Urinary Antigen Test (additional pneumonia test)
Not recommended for most people
Use it only in severe cases, like ICU patients with very sick CAP
It looks for a piece of the Streptococcus pneumoniae cell wall in the urine
It helps check if S. pneumoniae (the most common CAP bug) might be the cause of pneumonia
High specificity (>94%)
If the test is positive, it’s probably real
Varied sensitivity (>65%)
If the test is negative, it might still miss some true cases
It works best when the patient has bacteremia (bacteria in the blood), that boosts the test's reliability
Even if the test is positive, doctors usually still give the same antibiotics, so it doesn’t change the treatment plan much
That’s why it’s not recommended routinely, except maybe in severe cases (ICU)
Legionella Urinary Antigen Test (additional pneumonia test)
Not recommended for most people, except for:
Severe pneumonia (like in ICU)
Epidemiologic clues, like:
Local outbreak
Travel to hotel, cruise, etc. (think contaminated water systems)
Is a urine test that looks for antigens (proteins) from Legionella pneumophila, the bacteria that causes Legionnaires’ disease, a type of severe pneumonia
Detects only serotype 1
Serotype 1 is the most common cause of Legionella pneumonia (~84% of cases)
So this test misses other serotypes, that’s a limitation
99% specificity (damnnn)
The test may stay positive for weeks to months, even after the patient is cured
So it's not useful to monitor response to treatment, just good for diagnosis
Legionella Pneumophila
A bacteria that causes a serious type of pneumonia called Legionnaires’ disease
Becoming more common in recent years
Usually comes from environmental water sources:
Like hot tubs, cooling towers, or air conditioning systems
Symptoms:
Muscle aches
Dry cough (non-productive)
High fever
Gets worse fast
Chest x-ray shows patchy infiltrates or nodular infiltrates (uneven or spotty infection, not a classic pattern)
These patients are often very sick and may need hospitalization, even ICU care
Aspiration Pneumonia/Pneumonitis
Aspiration is something other than air (like food, saliva, vomit) accidentally goes into the lungs
Most often seen in:
Elderly patients
People from nursing homes or long-term care
Hard to tell the difference:
Pneumonia = infection
Pneumonitis = inflammation without infection
You don't need to add more antibiotics (like anaerobic medication (anaerobes already in mouth)) just because a patient aspirated
Stick with the normal pneumonia meds, unless there’s a clear reason to go bigger
Pneumonia
Infection
Pneumonitis
Inflammation without infection
Treatment Duration for CAP
5–7 days
You can stop antibiotics earlier if:
The patient is afebrile (no fever) for 48–72 hours
Their symptoms are improving
CAP Newly Approved Medication: fluoroquinolones, macrolides (these should not exceed 5 days)
Some patients with mild CAP might do well with just 3 days of antibiotics if they respond quickly
Guidelines say: Max 7 days, unless the patient isn’t improving
Shorter Treatment of CAP
A randomized controlled trial (RCT) studied 3 days vs 8 days of treatment.
Population studied:
Non-critically ill
Immunocompetent
Clinically stable after 3 days of β-lactam antibiotics
Result: No significant difference in outcomes (just waste of money to stay in hospital for that long)
Shorter Treatment of CAP Exclusions
Aspiration pneumonia
Legionella
Atypical pathogens
Lung abscess
Large pleural effusion
Directed Therapy vs Empiric Therapy
Empiric Therapy
You don’t know the exact bug yet, so you treat with broad coverage just in case
Directed Therapy
Once a specific bug is found (from cultures or tests), you narrow the treatment based on that bug’s susceptibility (what drugs it’s sensitive to) (so you know the bacteria already)
Most of the time, no pathogen is identified, so you just stick with empiric therapy
If you do find the bug → use directed therapy based on that organism’s resistance/sensitivity pattern
Directed Therapy for Legionella spp
Levofloxacin
OR
Azithromycin
Lefamulin Class
Pleuromutilin (new)
Lefamulin
Approved drug for CAP
Also Used for: S. aureus
Side Effects:
QT prolongation
Diarrhea
Cost:
Oral: ~$275/day
IV: ~$205/day
Dose Adjustment:
Decrease dose with reduced hepatic function
Omadacycline Class
Tetracycline
Omadacycline
Approved drug for CAP
Also Used for:
S. aureus
E. faecalis
Enterobacteriaceae
Other Indication:
SSTI
Side Effects:
Nausea/ vomiting
Increased LFTs
Cost:
Oral: ~$237/day
IV: ~$414/day
Dose Adjustment:
No dose adjustments
Delafloxacin Class
Fluoroquinolone
Delafloxacin
Approved drug for CAP
Also Used for:
S. aureus
P. aeruginosa
Enterobacteriaceae
Other Indication:
SSTI
Side Effects:
Nausea/ vomiting
FQ black box warning
Cost:
Oral: ~$180/day
IV:~$319/day
Dose Adjustment:
Decrease dose with reduced renal function (IV only)
Role for New Drugs for CAP
Not in severe CAP
These drugs were tested mostly in milder pneumonia cases, where patients didn’t need to be admitted to the hospital or ICU
Most helpful in PORT Class IV or lower (moderate risk)
Non-β-lactams
Great option if a patient is allergic to β-lactams
Effective against common pneumonia-causing bacteria
But they are much more expensive than the usual antibiotics
Bacterial CAP most commonly caused by…
S. pneumoniae
HAP
Pneumonia that develops ≥48 hours after hospital admission (not present at admission)
Intubation as a result of developing HAP is NOT a VAP
Pneumonia developing < 48 hours after admission is CAP
VAP
Pneumonia that develops ≥48 hours after intubation
HAP / VAP
Most common hospital-acquired infection:
Makes up 22% of all hospital-acquired infections
10% of ventilated patients get VAP
Serious impact:
Associated with high morbidity and mortality
Patients stay longer on the ventilator, longer in the hospital, and it costs more
Pathophysiology of VAP
Colonization of the upper respiratory tract
Bacteria build up in the mouth, throat, or trachea (often from the hospital environment)
Host defenses are bypassed
Normally, your upper airway (like the nose and throat) filters out bacteria
But when a ventilator (endotracheal tube) is inserted, that defense is skipped
Mucociliary clearance is also impaired
Bacteria pass through or around the endotracheal tube
Microorganisms can:
Travel down inside the tube
Or leak around the cuff that’s meant to seal the airway
HAP/VAP Diagnosis
Chest X-ray
But X-ray alone isn’t specific — it helps support the diagnosis, not confirm it alone
Quantitative Sampling
Samples from the lower respiratory tract are tested for bacterial load
There are cutoffs) to decide if the growth is significant or just colonization (like serious or just normal)
Signs and Symptoms of HAP/VAP
New or worsening sputum (mucus) production
New or worsening cough
Increased respiratory rate (breathing faster)
Pleuritic chest pain (pain when breathing in)
Decreased oxygenation (patient needs more oxygen or has lower oxygen levels)
Fever or hypothermia (low temp)
Leukocytosis or leukopenia
Lung Sounds (auscultation)
Crackles or rales
Changes in Ventilator Settings
Increasing FiO₂
Increased RR
Worsening respiratory acidosis (more CO2 in blood)
Non-Invasive Sampling Type (HAP/VAP)
Sputum
Patient coughs up mucus for testing
Tracheal aspirate
Diagnostic threshold: >10⁵ CFU/mL
Means infection is likely if bacteria grow at this level
Invasive Sampling (HAP/VAP)
Bronchoalveolar Lavage (BAL)
Diagnostic threshold: >10⁴ CFU/mL
miniBAL / non-bronchoscopic BAL
Diagnostic threshold: >10⁴ CFU/mL
Protected specimen brush
Diagnostic threshold: >10³ CFU/mL
Bronchoalveolar Lavage (BAL)
Quantitative culture method used to:
Collect fluid from deep inside the lungs
Measure bacteria levels to help diagnose pneumonia
A bronchoscope (thin tube with a camera) is inserted through the nose or mouth
Sterile fluid is squirted into a small part of the lung
The fluid is suctioned back and sent to the lab for culture
Looks for the amount and type of bacteria present.
Threshold for infection is usually >10⁴ CFU/mL
mini-BAL (non-bronchoscopic BAL)
A blind version of BAL (no camera used)
Still collects fluid, but less precise because it doesn’t visualize the lungs
Sampling Recommandation for HAP/VAP
Routine invasive sampling is no longer recommended for VAP
Guidelines now say don’t do invasive sampling automatically
Why?
Studies show no major difference in outcomes between invasive (e.g., BAL) and non-invasive (e.g., sputum, tracheal aspirate) methods
More strong studies are still needed
If you do use invasive sampling (like BAL):
Only treat with antibiotics if the bacterial count is above the diagnostic threshold (e.g., >10⁴ CFU/mL for BAL)
If below threshold = don’t treat, because it's likely colonization, not infection
Non-invasive sampling is fine and useful
Results from sputum or tracheal aspirates should be used to guide antibiotic choices
Biomarkers for HAP/VAP
Procalcitonin (sounds familiar…)
A precursor to calcitonin, but it rises specifically in response to bacterial infections (especially due to endotoxins)
Why it matters:
Helps differentiate bacterial from viral pneumonia
Can be used to guide when to stop antibiotics if levels drop
Has been studied the most out of the three listed
Use Procalcitonin for Starting Antibiotics for HAP/VAP?
No — avoid using it to start antibiotics
Should not replace clinical judgment (symptoms, CXR, vitals, etc.)
Studies used different cutoffs, making it unreliable for deciding when to start treatment
Use Procalcitonin for Stopping Antibiotics in HAP/VAP?
Yes, it may help guide when to stop antibiotics
Good for helping prevent overuse of antibiotics
VAP studies show that using procalcitonin to guide stopping therapy:
Reduced treatment duration (from 12.1 to 9.1 days)
No increase in failure or mortality
Nosocomial Pneumonia Bacteria
Staphylococcus aureus
Pseudomonas aeruginosa
Klebsiella pneumoniae
E. coli
Enterobacter spp.
Acinetobacter baumannii
Empiric Antibiotic Therapy for HAP/VAP
Guided by Local Susceptibilities
Use your hospital’s antibiogram (especially VAP-specific if available)
This shows how common bacteria respond to antibiotics in your setting
Coverage Should Include:
Staphylococcus aureus
Pseudomonas aeruginosa
Other Gram-negatives
When to add MRSA coverage to empiric therapy in HAP/VAP patient
IV antibiotics in the past 90 days
High risk of mortality (e.g., septic shock, or on the ventilator for HAP)
Unit MRSA rate > 20%
Empiric MRSA for VAP
Hospitalized for ≥5 days
Had ARDS before VAP
On dialysis/renal replacement therapy before VAP started
MRSA Pneumonia Agents
Vancomycin
15 mg/kg IV every 8–12 hours
Add a loading dose (25–30 mg/kg once) for severely ill patients
Linezolid
600 mg IV every 12 hours
Vancomycin vs. Linezoild
Prospective, double-blind, multicenter
448 patients
Compared:
Linezolid 600 mg IV q12h
Vancomycin 15 mg/kg IV q12h, goal trough 15–20 µg/mL
Mortality: No significant difference (15.7% for linezolid vs 17% for vancomycin).
Clinical cure: Higher in the linezolid group (57.6% vs 46.6%) — this was statistically significant
Kidney safety: Linezolid caused less kidney damage (nephrotoxicity) than vancomycin (8.4% vs 18.2%)
Empiric Gram-Negative Coverage
Goal:
Ensure activity against Pseudomonas, a tough gram-negative bug often found in hospital infections
Adjust coverage if the patient has risk factors like:
Septic shock or ventilator support (high mortality risk)
Received IV antibiotics in the last 90 days
VAP-specific risks, such as:
Local resistance >10%
ARDS (acute respiratory distress syndrome)
On dialysis or other renal replacement therapy
Hospitalized ≥ 5 days
Antibiotic Options for Pseudomas (Antipseudomonals!!)
Pip-Tazo (anti-pseudomal pen)
Cefepime or Ceftazidime – both are cephalosporins, but:
Cefepime covers MSSA
Ceftazidime does NOT cover MSSA
Imipenem or Meropenem
Aztreonam (does NOT cover MSSA)
Who needs combo therapy?
Patients with:
High risk of mortality (e.g., septic shock)
Risk factors for MDR organisms (like previous antibiotic use, prolonged hospitalization)
Combination therapy for gram-negative infections
Ciprofloxacin or Levofloxacin
Amikacin or Gentamicin or Tobramycin
Colistin or Polymyxin B
So you combine these, with:
Pip-Tazo (anti-pseudomal pen)
Cefepime or Ceftazidime – both are cephalosporins, but:
Cefepime covers MSSA
Ceftazidime does NOT cover MSSA
Imipenem or Meropenem
Aztreonam (does NOT cover MSSA)
Empiric Treatment: HAP
Did the patient develop pneumonia ≥ 48 hours after hospital admission?
If yes → move to next step. (This makes it HAP, not CAP.)
Does the patient have any of these high-risk factors?
Septic shock
Ventilator support
Received IV antibiotics in the last 90 days
If YES → Give MRSA coverage + Double Gram-negative coverage
If NO → go to step 3
Is the local MRSA rate > 20%?
If YES → Give MRSA coverage + Antipseudomonal β-lactam
If NO → Just give an antipseudomonal β-lactam
But make sure it also covers MSSA, not just Pseudomonas
Empiric Treatment: VAP
Did the patient develop pneumonia ≥ 48 hours after incubation admission?
If yes → This is VAP
Are there any of the following high-risk factors?
Septic shock
IV antibiotics used within the past 90 days
Hospitalized ≥ 5 days before VAP onset
Acute respiratory distress syndrome (ARDS)
Acute renal replacement therapy (ARRT)
If YES → Go to Step 3
If NO → Go to Step 3B
Step 3A: (High-Risk Patients)
MRSA Coverage + double antipseudomonal coverage
Step 3B: (Lower-Risk Patients)
You can consider just 1 Gram-negative drug and skip MRSA coverage only if:
Pseudomonas resistance to single agents is <10%
MRSA prevalence is <10–20%
If your hospital antibiogram shows low resistance, use:
A single β-lactam that covers MSSA and Pseudomonas
(like pip/tazo, cefepime, meropenem)
How long should you give antibiotics for VAP?
7 days
BUT...
If the VAP is caused by non-fermenting gram-negative bacilli (NF-GNB) like:
Pseudomonas aeruginosa
Acinetobacter baumannii
Stenotrophomonas maltophilia
These are harder to kill and more likely to come back — so 7 days might not be enough
But if they are improving stick the 7 days
Inhaled Antibiotics in VAP
Use adjunctive inhaled antibiotics (in addition to IV)
Only if the bacteria causing the infection are:
Only treatable by aminoglycosides (like gentamicin or amikacin)
OROnly treatable by polymyxins (like colistin or polymyxin B)
So: If regular IV antibiotics won’t work and the only antibiotics that work are the ones you can also give through inhalation, then you can add inhaled versions to boost treatment.
But is inhaled therapy good enough on its own
Don’t use inhaled antibiotics alone — always pair them with IV antibiotics.
Meta-analysis (a big study that combines lots of studies) found:
More people had their infection go away (higher cure rate)
It did NOT lower the death rate
It did NOT increase kidney problems
So, it helps the infection clear up faster but doesn’t change whether someone survives or not