3 - Carbonyl Chemistry
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
81 Terms
nucleophilic substitution rxns:
exchanging an atom/substituent for another
exchanging a sigma bond for another sigma bond
nucleophilicity:
ability of a nucleophile to DONATE ELECTRON PAIR
more negative substituent = more nucleophilicity
electrophilicity:
the more POSITIVE, the better it accepts electron pair
What makes leaving group ability stronger?
the WEAKER the base, the BETTER it is at leaving b/c better stabilization by itself
SN2 rxn:
STRONG nucleophile (required) (backside) attack electrophile and LG, with LG leaving in ONE STEP
What happens to the product after SN2 attack?
inversion of configuration (S -> R, vice versa) due to nucleophile's backside attack
How to calculate rate law for SN2?
rate = k[E+][Nu-]
SN2 favors what kind of nucleophile?
small, STRONG nucleophile
SN2 favors what kind of substrate?
CH3 > 1° > 2°
SN2 works best in what kind of solvent?
polar, aprotic solvent (ex: acetone, DMF, DMSO)
Why is small and strong nucleophiles favored by SN2?
no bulky, easier backside attack
Why must SN2 rxns be performed in polar aprotic solvents?
b/c polar aprotic solvents CANNOT form H-bonds
What is rate dependent upon for SN2 rxns?
Nucleophiles only
How do alcohols and ethers undergo substitution rxns?
need initial ACID CATALYSIS step
Why do alcohols and ethers require acid catalysis to undergo substitution rxn?
-OH and -OR are BAD leaving groups (unstable on their own) but -OH2+ and -ORH+ are stable on their own (good LGs)
SN1 rxn mechanism:
creates a carbocation intermediate, and needs 2 steps
What is the rate-limiting step in SN1 rxn?
carbocation formation
What is the rate dependent on for SN1 rxn?
electrophiles only
SN1 rate law:
rate = k[E+][Nu-]
How do nucleophiles attack?
top/bottom attack to make TWO racemic products (enantiomers)
SN1 works best in what kind of solvent?
polar protic (ex: H2O, ROH)
What kind of nucleophile is used for SN1?
weak nucleophiles, solvent is often the nucleophile
SN1 favors what kind of substrate?
3° > 2°
why does SN1 favor only 3° and sometimes 2° substrates/electrophiles?
more steric hindrance will STABILIZE the carbocation intermediate
what is the product of alcohol oxidation?
aldehydes and ketones
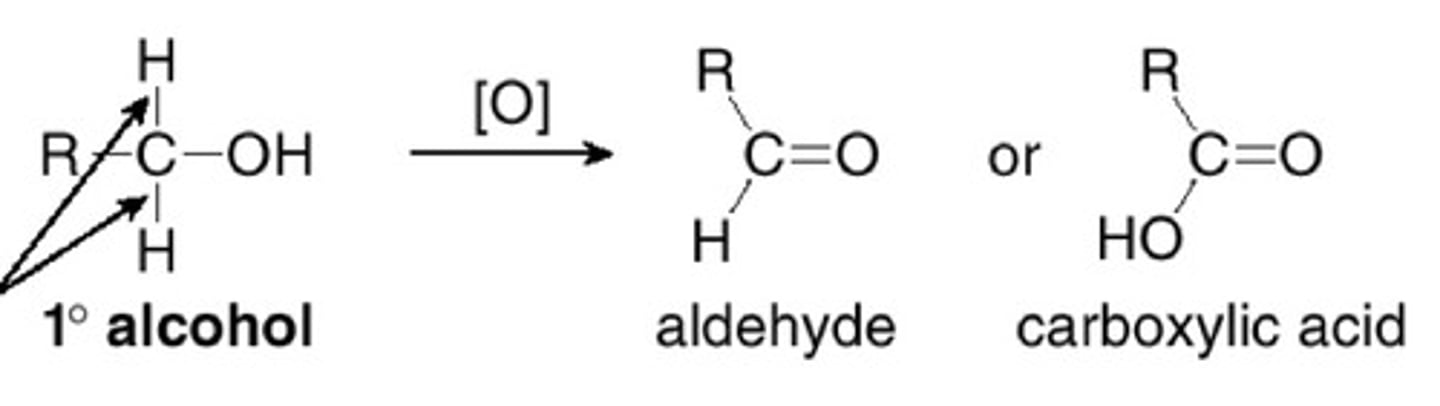
1° alcohol can turn into what w/ alcohol oxidation?
1° alcohol -> aldehyde -> carboxylic acid
2° alcohol can turn into what w/ alcohol oxidation?
ketones ONLY
3° alcohol can turn into what w/ alcohol oxidation?
NOTHING. they can't perform oxidation
Common oxidizing agents:
Mild - PCC
Strong - CrO3, MnO4
PCC:
mild oxidizing agent, can make aldehyde without furthering into 1° alcohol without turning it into COOH
Which carbon is acidic in a carbonyl?
alpha-carbon (adjacent to the carbonyl carbon)
Tautomerism:
equilibrium between keto (aldehyde/ketone) and enol forms of a molecule
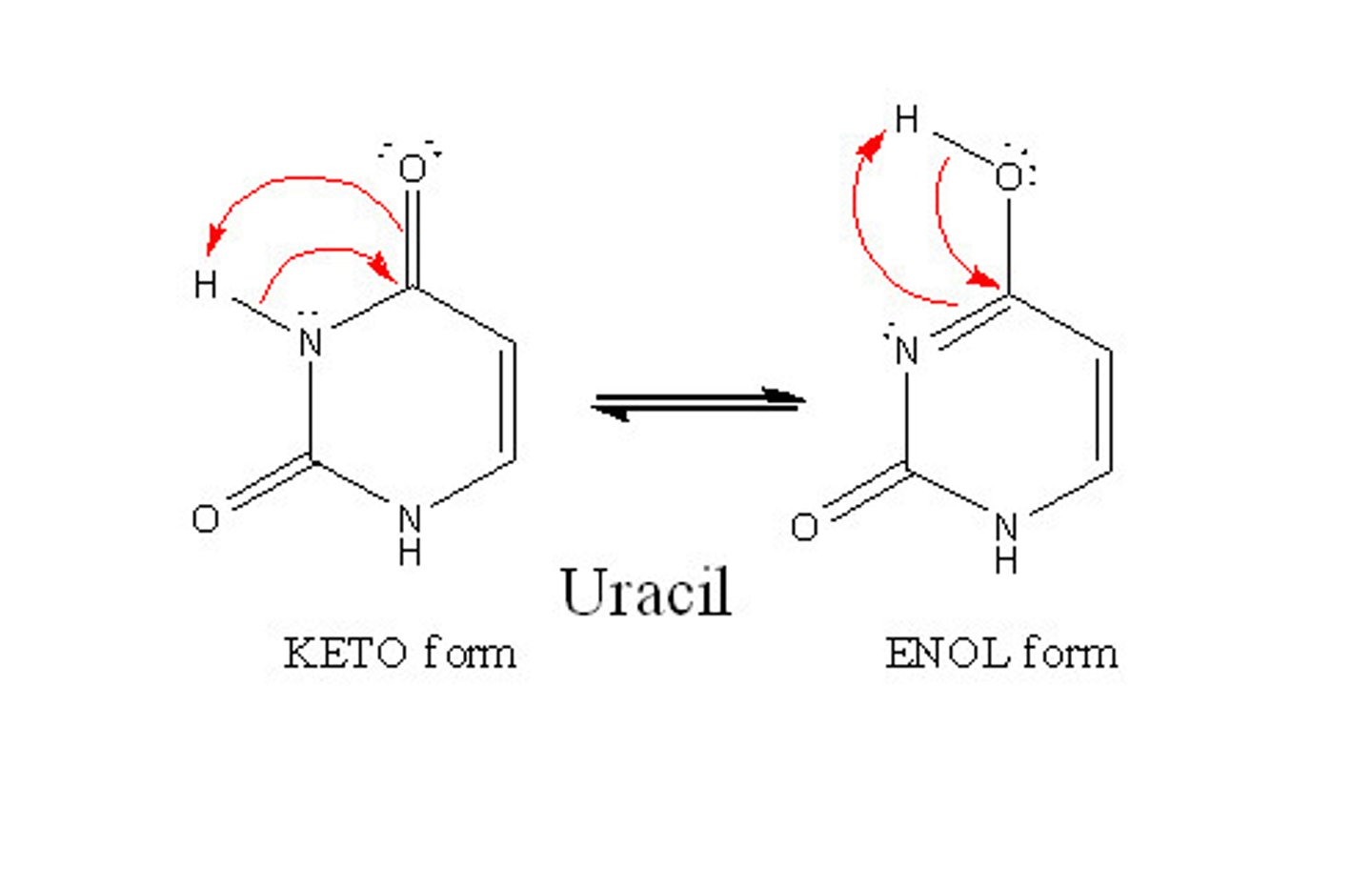
What is the relationship b/w keto and enol?
constitutional isomers
Which is more favored, keto or enol isomers?
keto b/c it is more stable
What is required to form enolates?
alpha protons
How many enolates can be formed with 2 alpha protons?
2 enolates
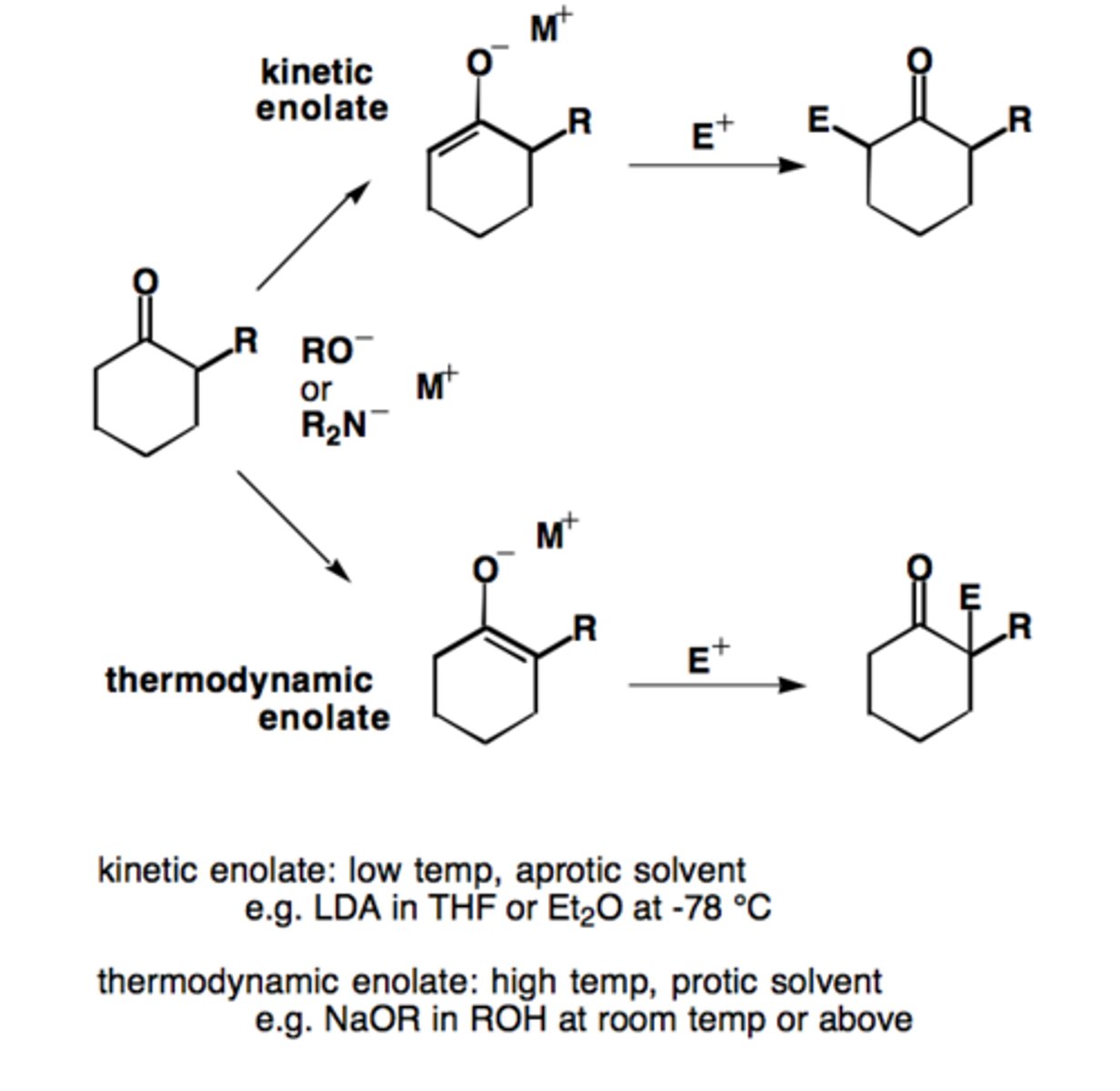
Thermodynamic enolate:
more substituted, more stable, and more favored
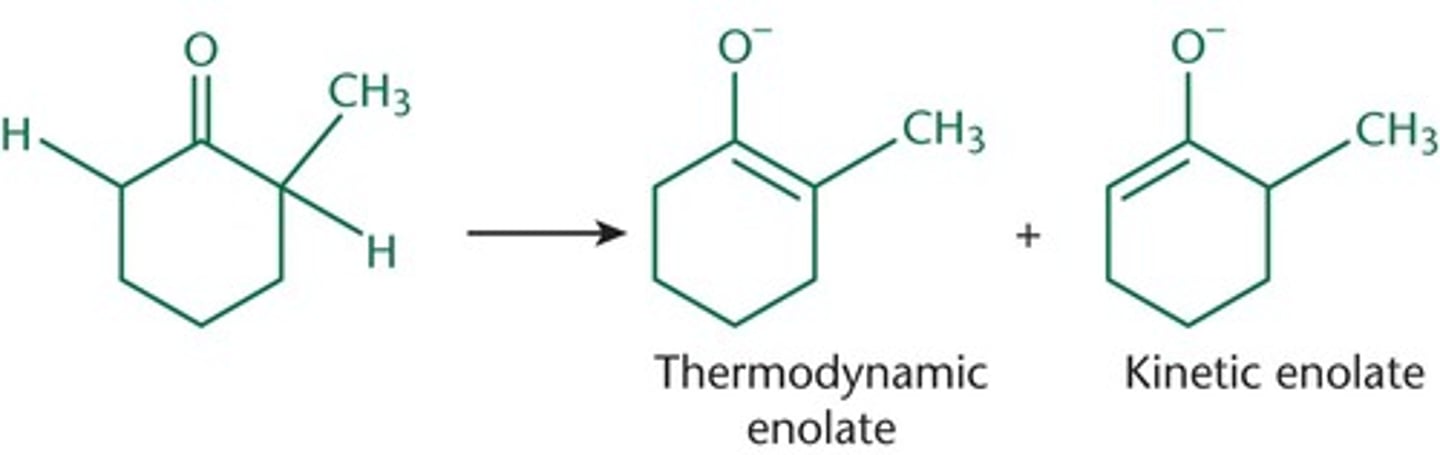
Kinetic enolate:
less substituted, less stable, forms faster
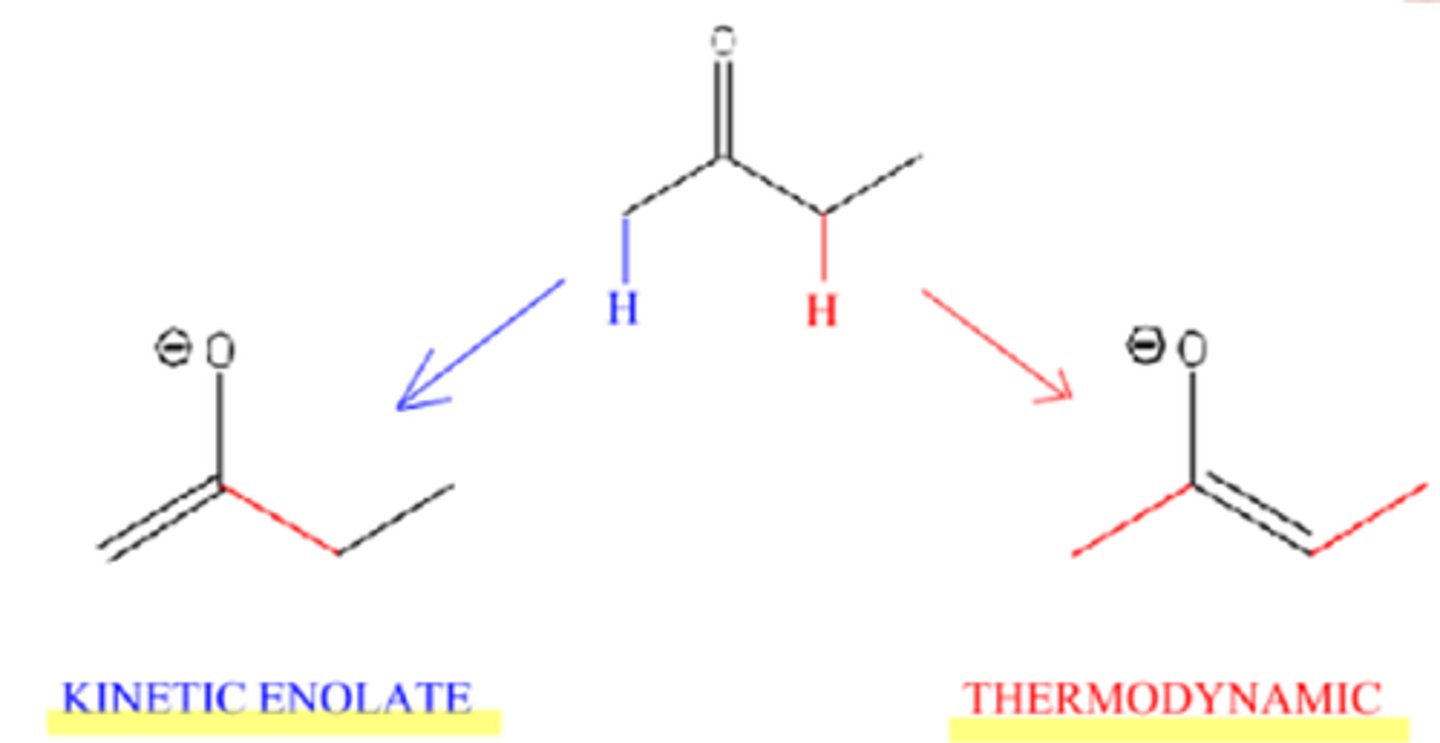
Conditions for thermodynamic enolate formation:
small base (-OR / -OH), higher temps (>25°C)
Conditions for kinetic enolate formation:
LDA (bulky base) , -78°C
Nucleophilic addition rxn:
lose 1 pi bond to gain 2 sigma bonds :D
Steps of nucleophilic addition rxn for STRONG nucleophiles:
1. nucleophile attacks carbonyl compound
2. acid workup (H3O+)
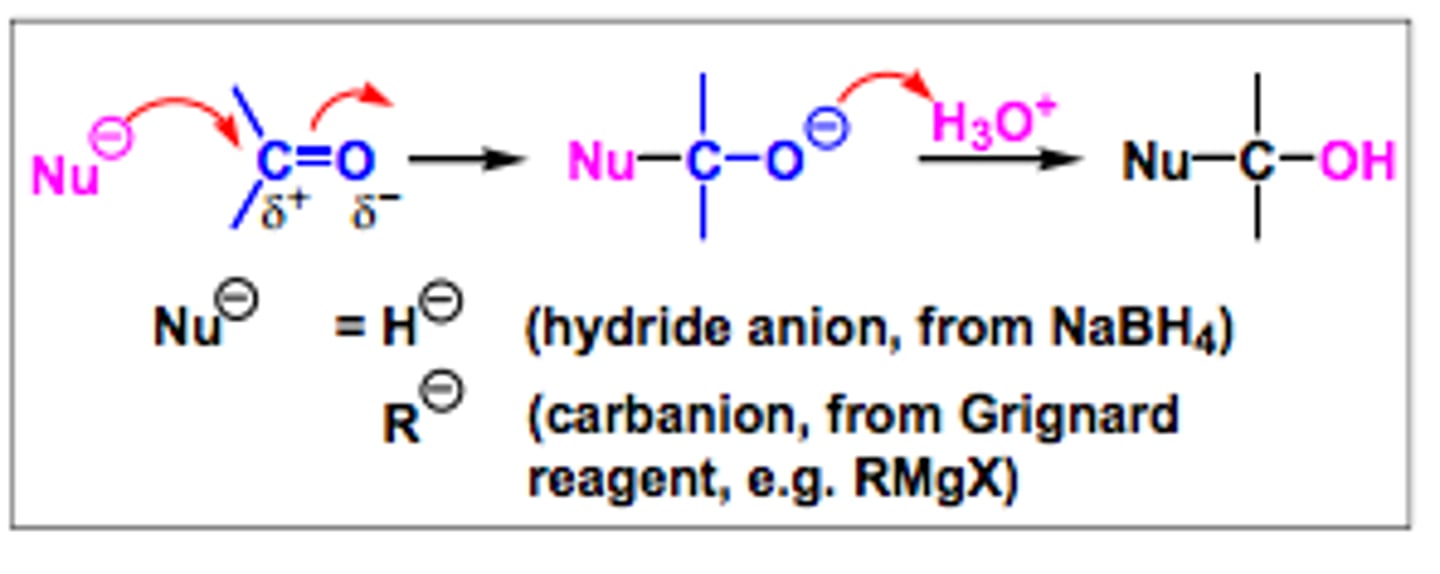
Steps of nucleophilic addition rxn for WEAK nucleophiles:
1. H3O+ protonates C=O
2. Nucleophile attacks carbonyl
Hydride rxns:
aldehydes/ketones turning into alcohols using reducing agents as hydride donors (H:-) and usually have boron (B) or Al
Steps in hydride rxn:
1. H:- attacks carbonyl
2. Acid workup (H3O+)
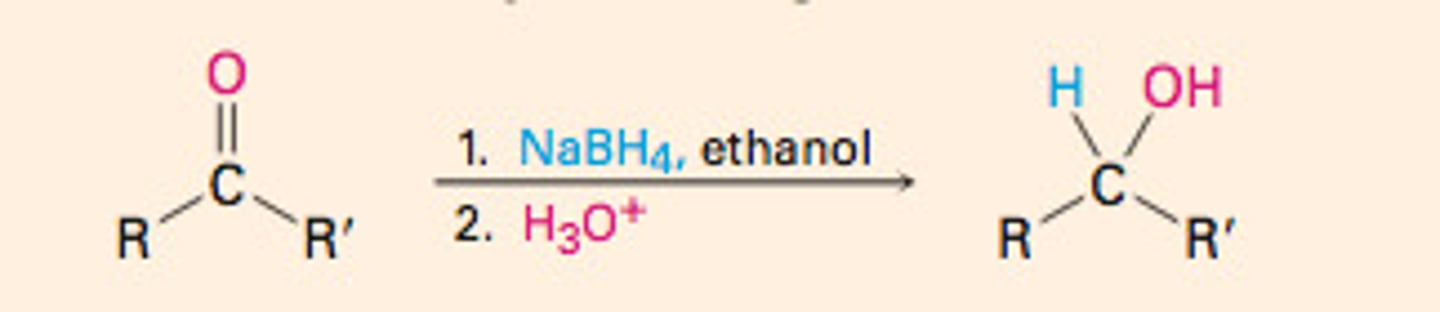
Can 3° alcohols be produced with hydride rxns?
No
Organometallic rxns:
adds a -CH3 group into a ketone/aldehyde + turn it into 3° alcohol (uses a grignard reagent -CH3MgX)
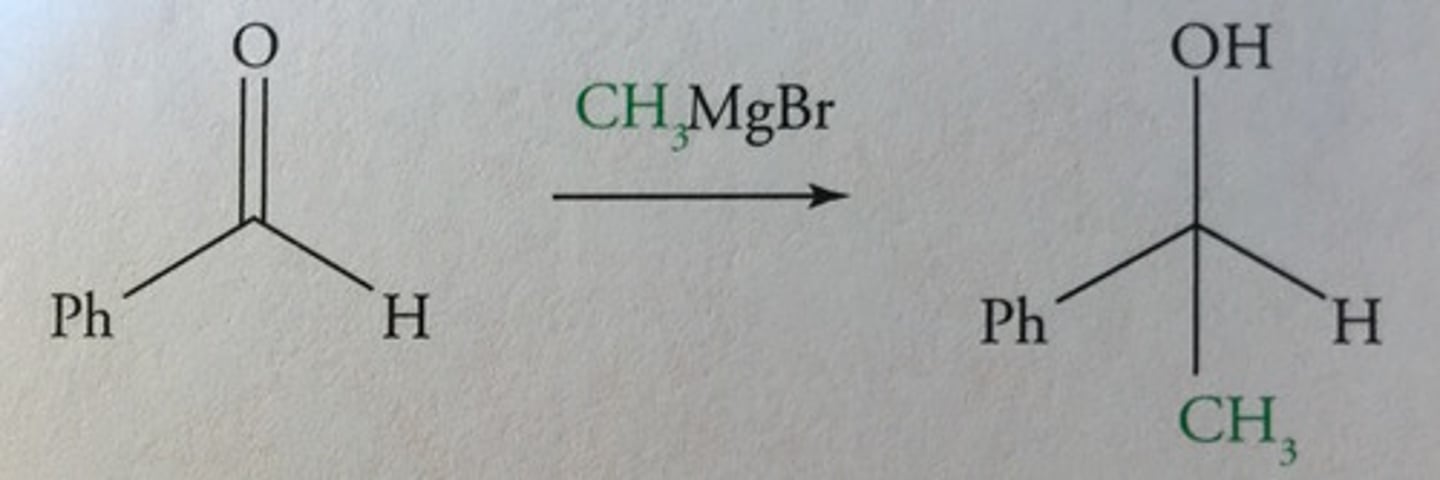
When are acetals/hemiacetals formed?
when ALCOHOL is the nucleophile (weak)
Hemiacetals:
1 OH and 1 OR group
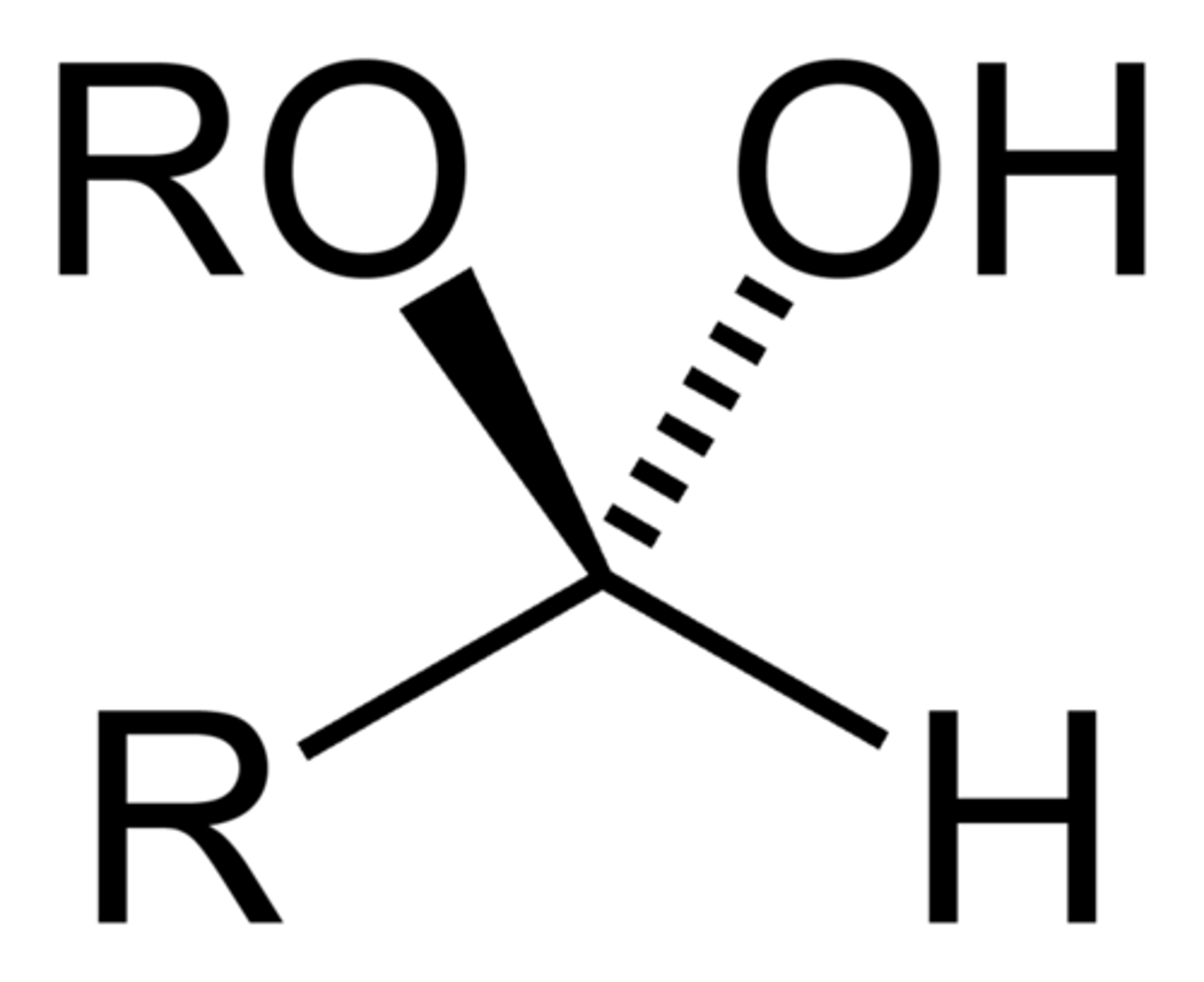
Hemi/acetals rxns:
aldehydes/ketones turn into hemi/acetal with R-OH
Acetals:
2 OR groups
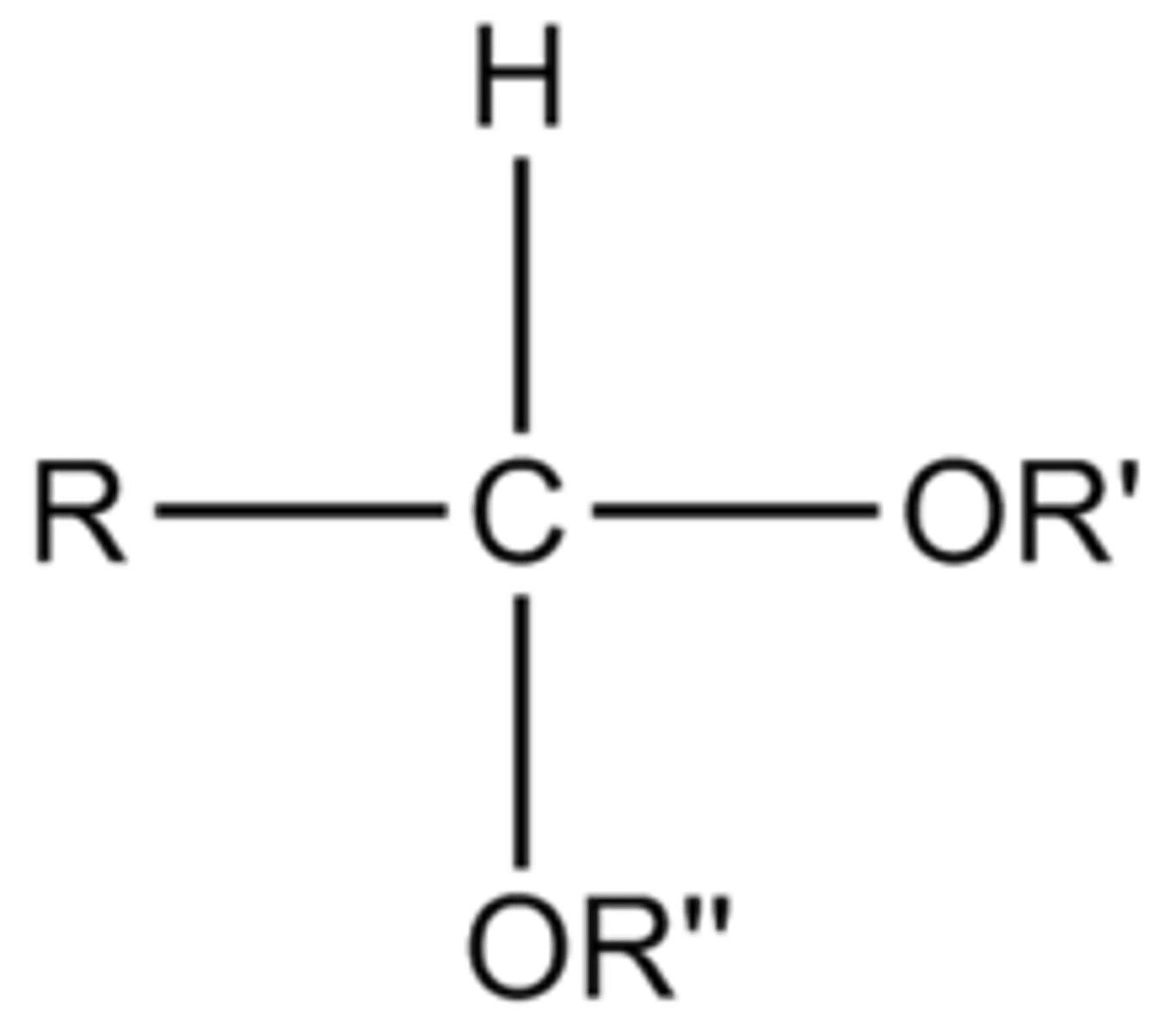
How to spot a hemi/acetal?
look for CARBON bonded to TWO -OR groups (acetal) or 2 OH/OR groups (hemiacetal)
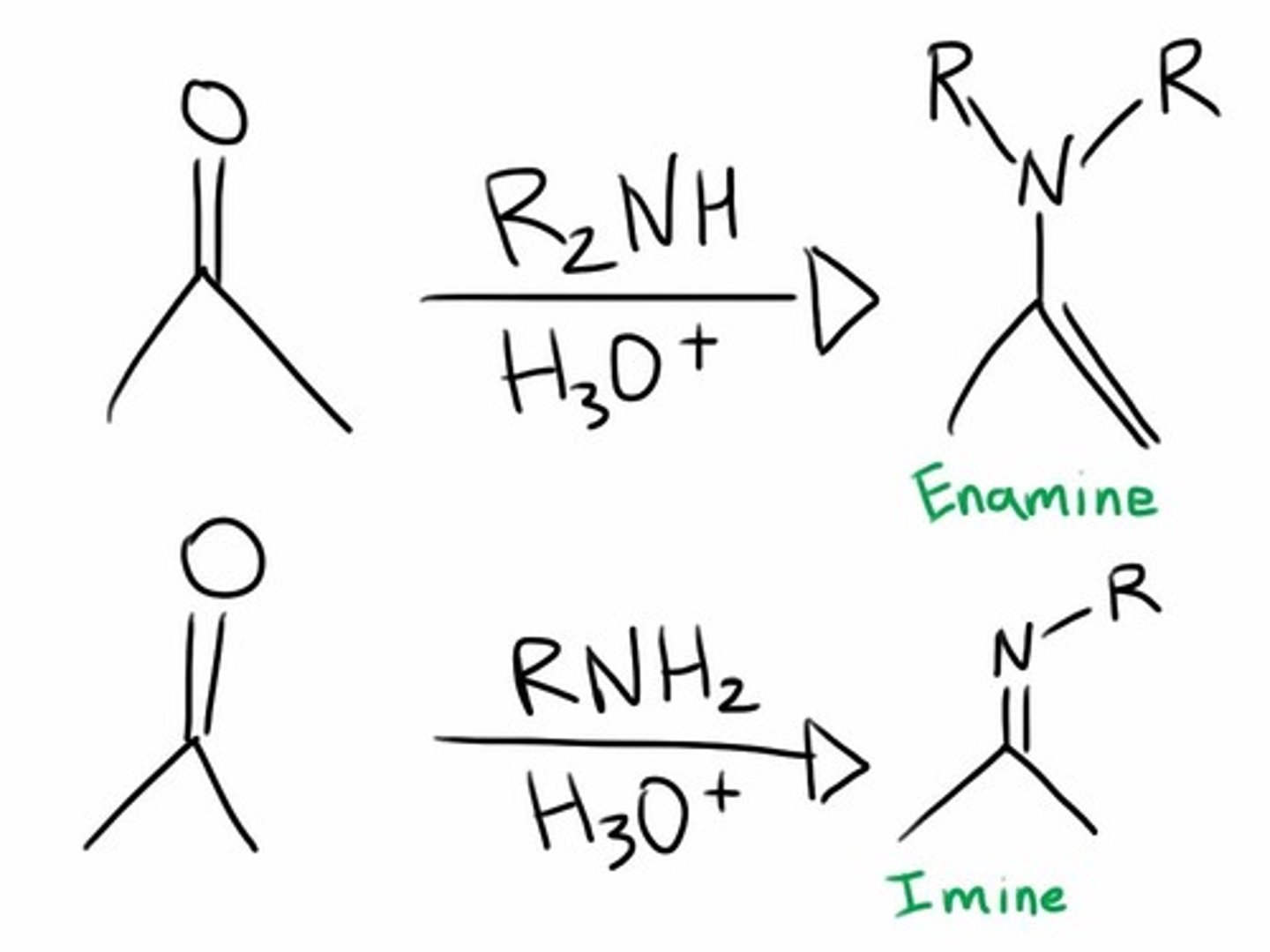
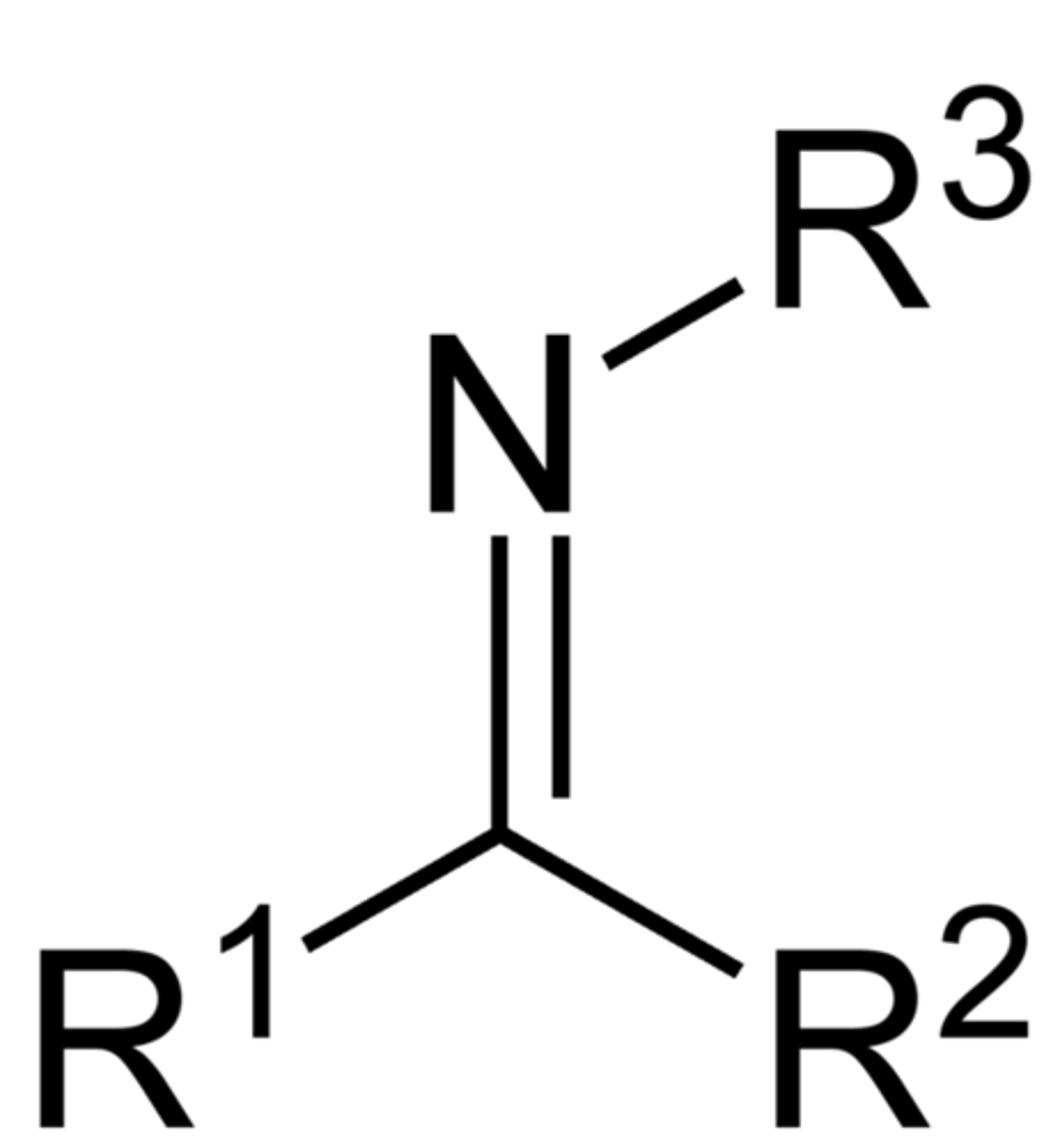
Imines:
form when a primary AMINE (RNH2) is the nucleophile (weak)
Imine structure:
Enamines:
form when a secondary amine (R2NH) is the nucleophile
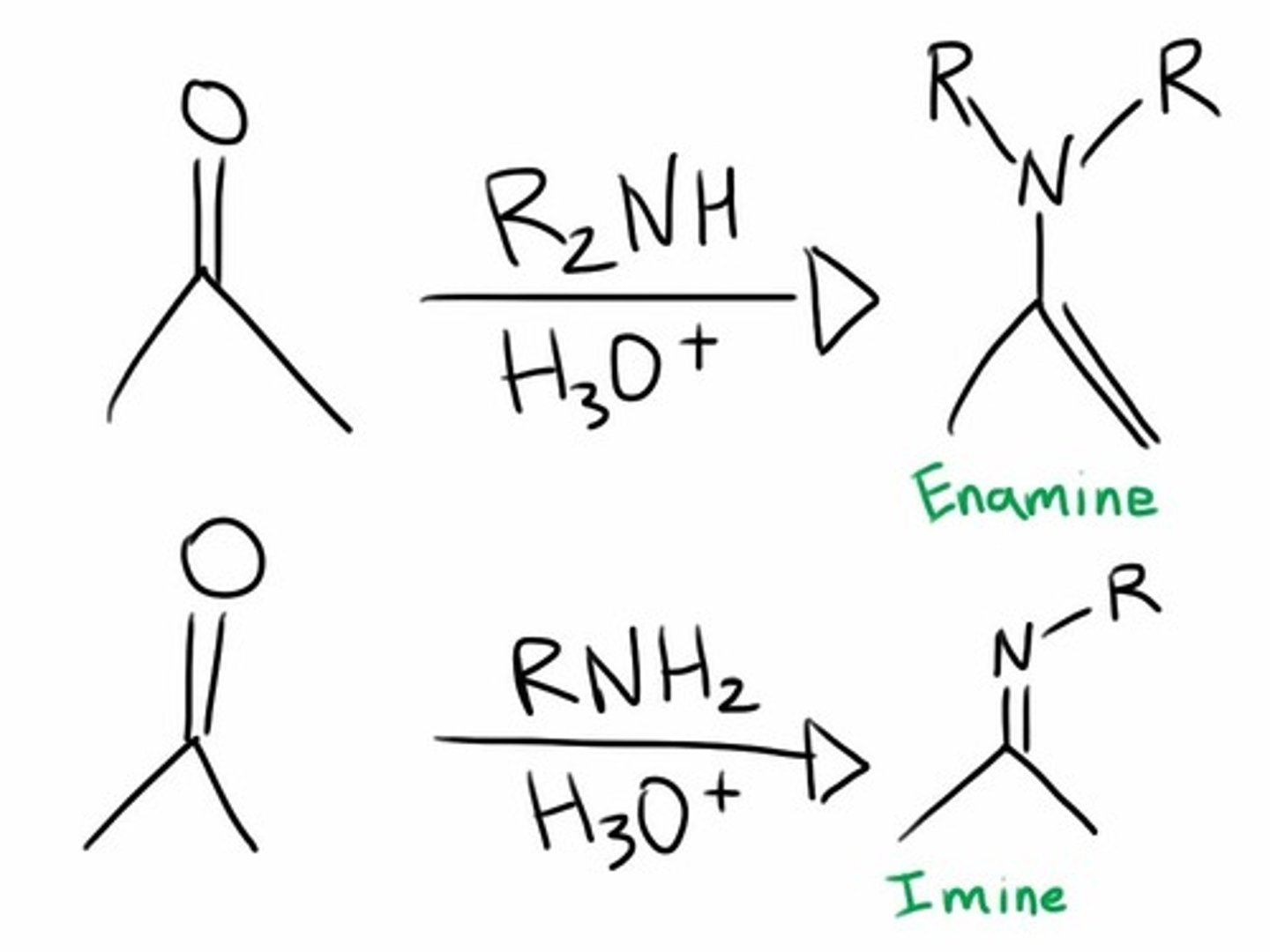
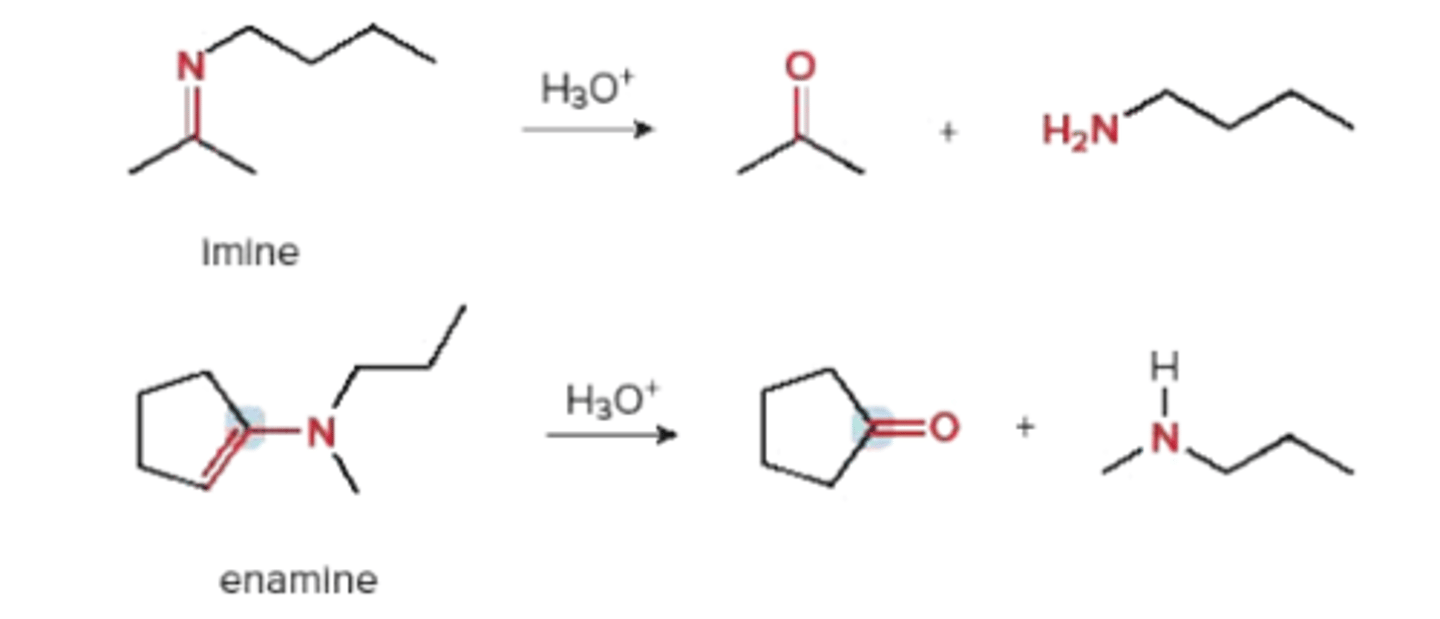
How to turn an enamine back to a carbonyl?
by adding water/H3O+ since enamines have NUCLEOPHILIC alpha carbons
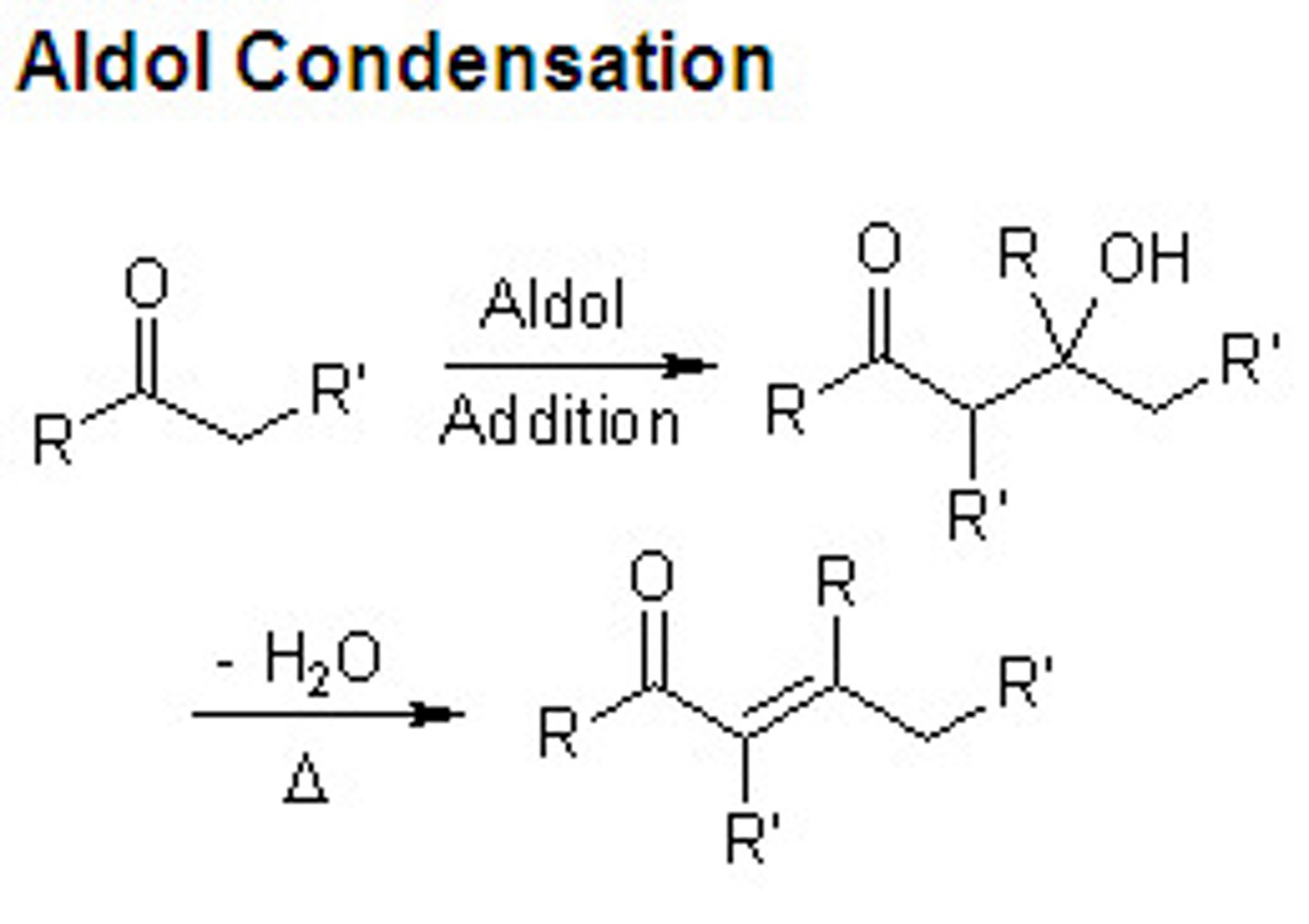
Aldol condensation:
when a nucleophilic enolate reacts with aldehyde/ketone to make an aldol product
Aldol formation
1. Enolate formation from aldehyde/ketone using a BASE (OH-)
2. Addition of enolate to carbonyl
What happens when heat and water added to the aldol product?
alcohol group leaves and an alkene is formed (IRREVERSIBLE)
retro-aldol rxn:
reverse of aldol rxn using acid or base
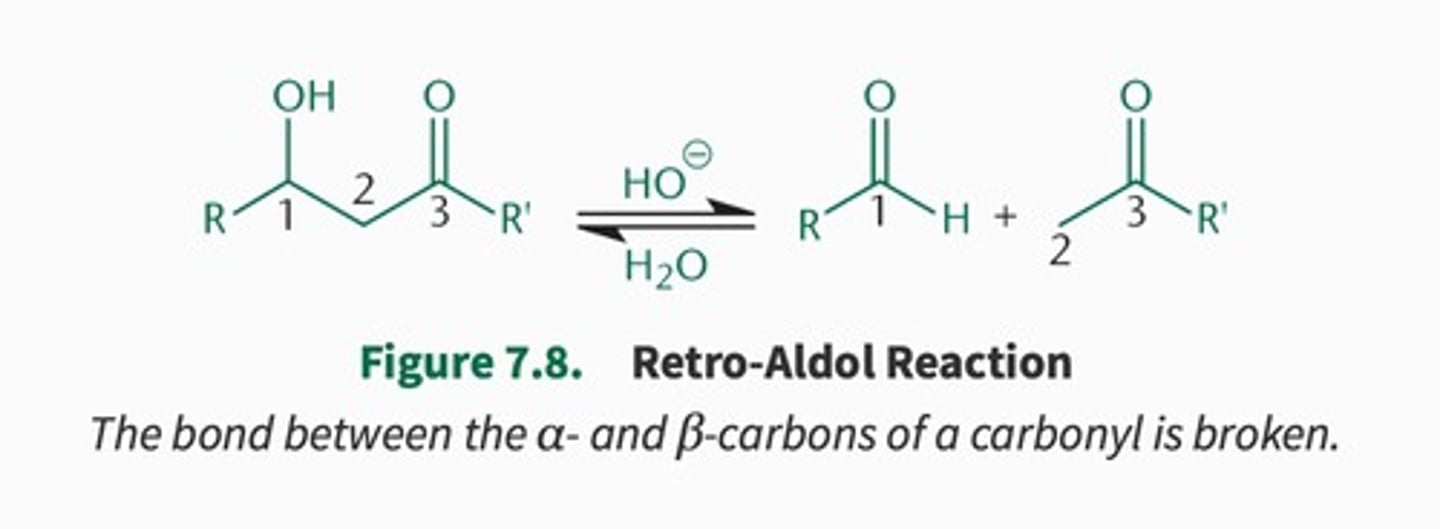
How to visualize the products of retro-aldol rxns?
locate the ALPHA and BETA bond and CUT between that
How to turn carboxylic acid into 1° alcohol?
1. LiAl4
2. H3O+
How are esters made?
carboxylic acids and alcohols through nucleophilic addition elimination rxn
nucleophilic addition elimination rxn:
1. addition step: nucleophile attacks carbonyl, carbon-oxygen pi bond breaks
2. elimination step: carbon-oxygen pi bond reforms, LG leaves
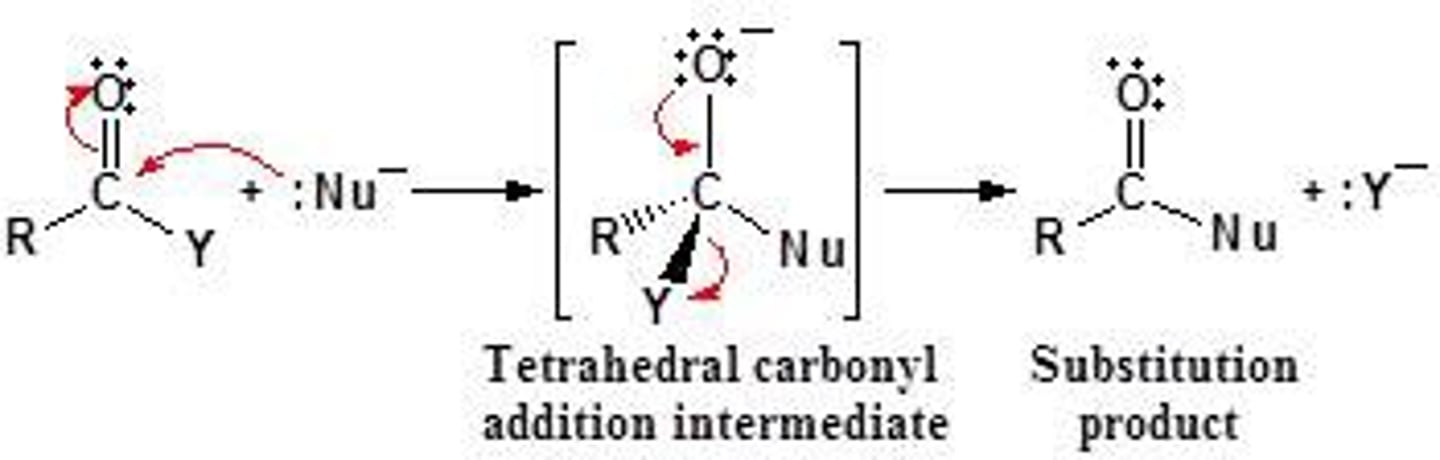
Ester hydrolysis:
reverse of ester formation
How are ester hydrolysis made?
any acidic conditions and water (to make back carboxylic acids and alcohol reactants)
Saponification rxn
hydroxide + ester to make carboxylate and alcohols
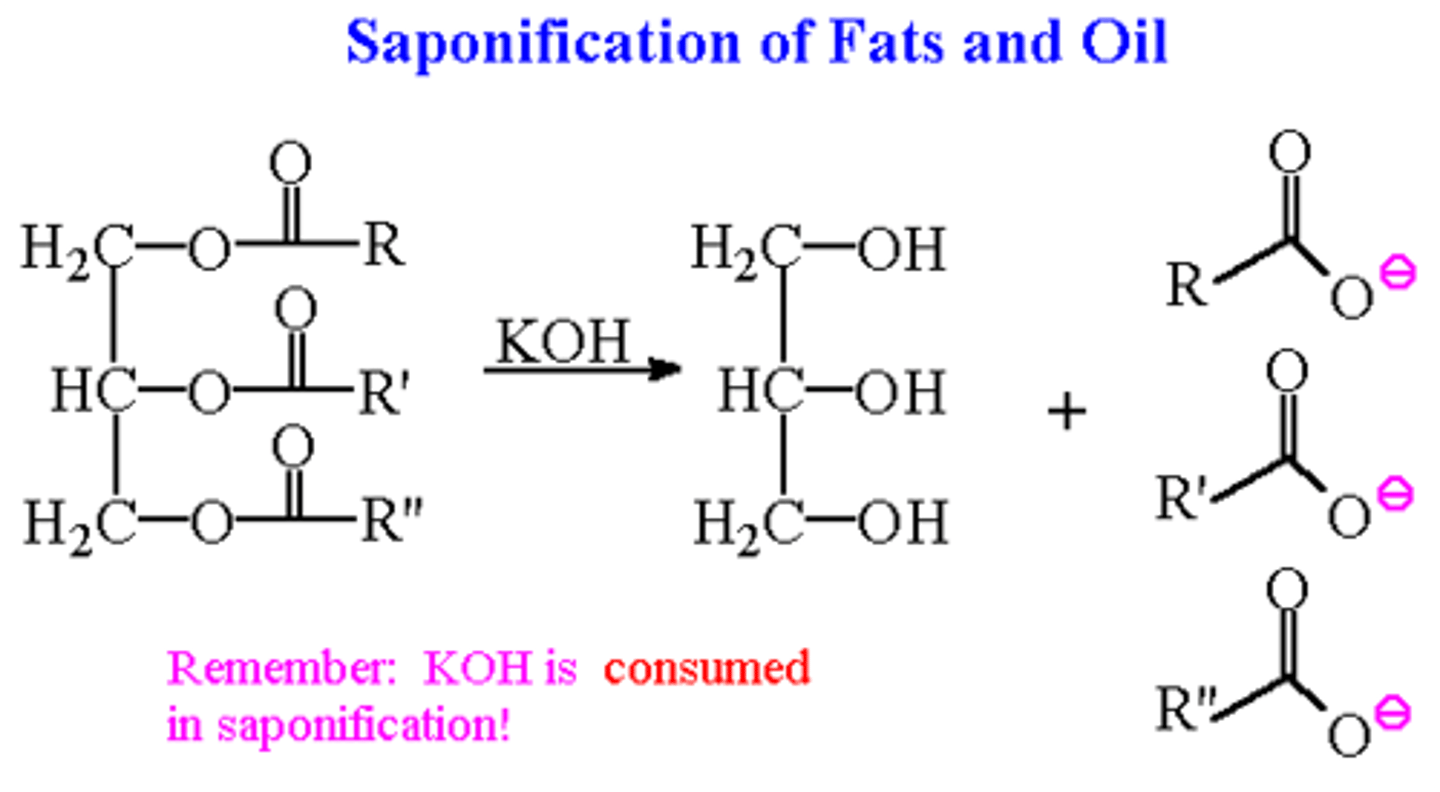
Why would carboxylic acid give its H to alcohol in saponification rxn?
alcohols are LESS acidic than carboxylic acids so H will go to alcohol
Rank in order of increasing carboxylic acid derivative reactivity:
acid anhydride, ester, acid chloride, amide
amide < ester < acid anhydride < acid chloride
Rank in order of increasing carboxylic acid derivative base strength:
acid anhydride, ester, acid chloride, amide
acid chloride < acid anhydride < ester < amide
Rank in order of increasing carboxylic acid derivative leaving group ability:
acid anhydride, ester, acid chloride, amide
amide < ester < acid anhydride < acid chloride
Which of the following carboxylic acid derivative can be made directly from C.A. herself?
A. acid halides
B. acid anhydride
C. ester
D. amide
A, B, C
Which of the following carboxylic acid derivative CANNOT be made directly by C.A. herself? Why?
A. acid halides
B. acid anhydride
C. ester
D. amide
D.
b/c amines (NH3) is MORE basic and would deprotonate alcohols (R-OH) instead of making carboxylic acid
Reactants to make acid halides (derivatives of C.A.):
C.A. and SOCl2 or PX3 (X = Cl, Br)
Reactants to make acid anhydrides (derivatives of C.A.):
acid chloride + carboxylate
Reactants to make esters (derivatives of C.A.):
acid anhydride/acid chloride + alcohol
What are some STRONG typical nucleophiles that will attack a carbonyl C?
- hydride (H-): adds an alcohol and makes a new pi bond, remove 1 carbonyl sigma bond
- grignard (R-): adds an R group and makes a new pi bond, removes 1 carbonyl sigma bond
- enolates: tautomerization rxns
what are some WEAK typical nucleophiles that will attach a carbonyl C?
- alcohol (-ROH): SN1 rxn
- amines (1°, RNH2; and 2°, R2NH): imine formation, enamine formation
if the nucleophile is WEAK, what must accompany that nucleophile to complete the rxn?
acid catalysis! (H3O+) to make a better carbonyl leaving group
under what condition does elimination follow addition? (for nucleophilic addition elimination rxn)
a good leaving group must be attached to carbonyl C post-addition rxn
What happens to nucleophilic addition elimination rxn if there is no good LG?
the rxn will be addition ONLY