Biology
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
polar molecule
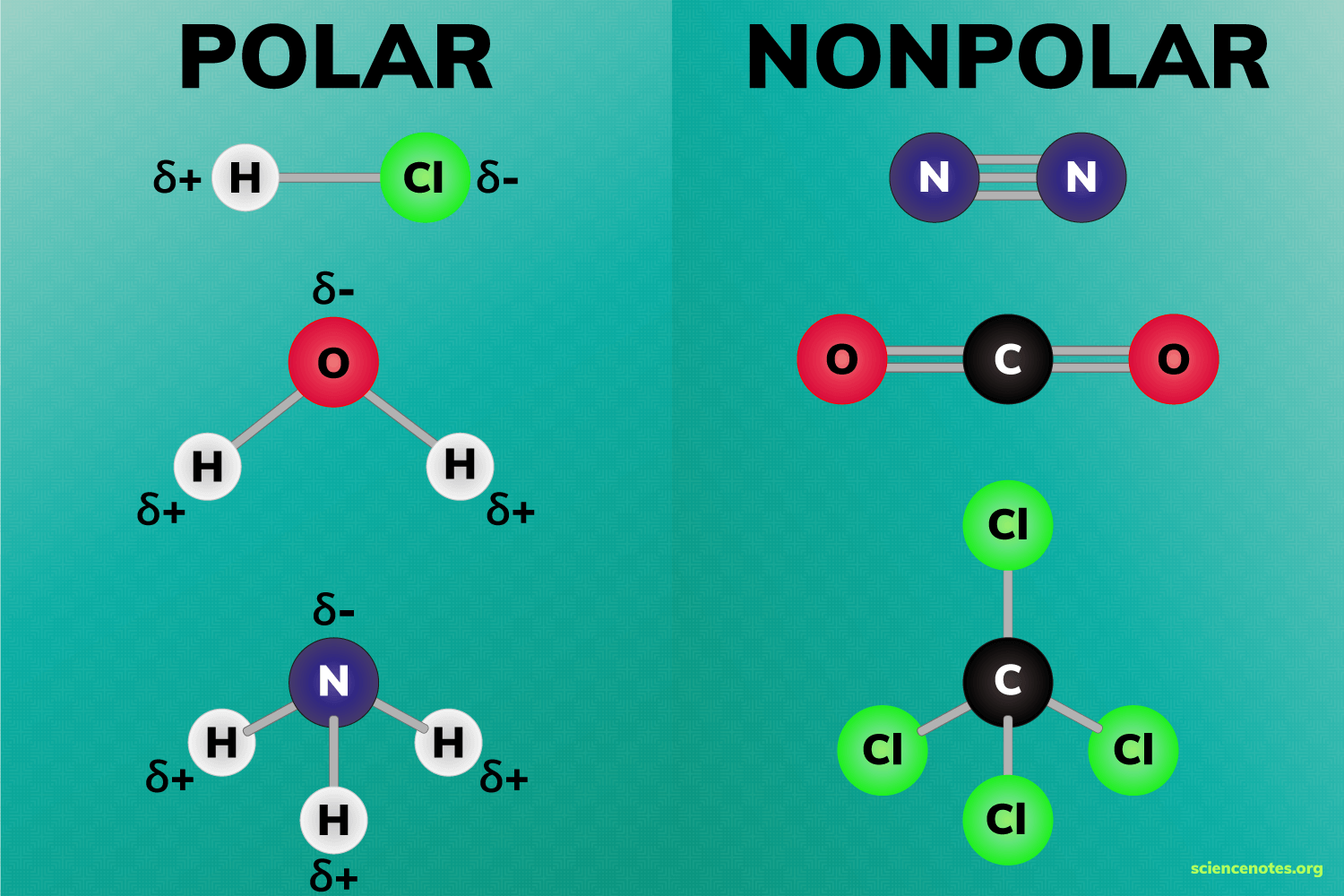
has an uneven distribution of charge, both positive and negative regions due to difference in electronegativity Amon atoms
chemical formula
represents the proportion of atoms per element that make up a chemical compound using symbols from the periodic table. water is made up of two hudrgoens and one oxygen. it is a polar molecule.
what are polar substances attracted to
other polar substances, just as non polar substances are attracted to other non polar substances. polar and non polar do not attract because they are not alike.
hydrophilic substance
attracts water, has an affinity for water
hydrophobic substance
avoids water and does not have an affinity for water. lipids are hydrophobic and have relatively non polar bonds
hydrogen bonding
when an intermolecular bond that occurs because of a hydrogen atom bonding to a highly electronegative atom (oxygen, nitrogen, fluorine)
electronegative atom
attracts shared electrons in a covalent bond towards itself
dipole moment
is a measure of the polarity of a molecule, and indicates how much a positive and negative charge is separated within the molecule
dipole moment is created in the molecule when
the hydrogen carries a partial negative charge, the type of structure we observed that gives water its polarity
water carries a…
partial positive charge - occurs when an atom shares its electron unequally with another atom in covalent bonding. The atoms that pulls less strongly on shared electrons had a partial positive charge due to a lack of electron density
electronegative atom give water…
its polarity. It has a partial negative charge as well
hydrogen bonds are…
weaker than covalent bonds but they are still relatively strong and play an important role in many chemical and biological processes
why is hydrogen bonding critical?
it is responsible for many molecular properties, such as shape and function of proteins, stability of many chemical compounds, and the formation of intermolecular interactions in crystalline solids.
what do hydrogen bonds between water molecules give water properties of?
cohesion - tendency for particles or molecules of the same substance to stick together, attraction of similar kinds of molecules. it’s like being drawn to people who look like you at that same party
adhesion - attraction between different types of molecules
capillary action - when water moves up against gravity through small tubes due to adhesion and cohesion
surface tension - the cohesive force at the surface of a liquid that allows it to resist external forces, water molecules sticking together create surface tension
specific heat - refers to the amount of heat energy required to raise on gram of substance by one degree celsius. heat capacity is the total amount of heat energy required to raise the temperature of an entire object, not just one gram. it’s like how much water it would take to completely saturate a sponge
evaporative cooling - process where surface of an object becomes cooler during evaporation. this occurs because molecules with the highest kinetic energy are the most likely to evaporate, leaving behind molecules with lower average kinetic energy and this reducing tempreautre
cohesion
contributes to the transport of water and nutrients against gravity of plants
transpiration
the loss of water from. plant in the from of water vapor
adhesion
when one substance is attracted to another. water adhered ot other molecules and surfaces
surface tension
difficulty ot break surface of water because of cohesive forces
water has what type of specific heat
water has a high specific heat, so it can absorb or release a large amount of heat with only a slight change in its own temperature so large bodies of water take a while to evaporate
evaporative cooling
water had a high heat of vaporization, so the water can absorb a lot of heat and leave the surface cooler example; extra body heat is used to convert beads of meat/persipitation into vapor, whihc cools down the body.
dissociation of water
hydrogen shifts from one water molecule to another. h20 molecules are broken down into ions- specifically, one hydrogen ion h+ an on hydroxide ion oh-
hydronium concentration
is the measure of the amount of hydronium ions in a solution, it is used to determine the acidity or alkalinity of a ph solution
hydrogen ions
are single proton with no electrons and play crucial roles in chemical reactions, especially those involving acids and bases
when a molecule is increasing hydronium concentration by releasing hydrogen ions into solutions it is an…
acid (any substance that donate protons or hydrogen ions from an aqueous solution, which decreases hydronium ion concentrations
when a molecule is increasing hydroxide concentration by abosrbing or accepting hydrogen ions…
it is a base (substance that can accept protons or donate a pair of valence electrons, have a PH value greater than seven)
ph scale
logarithmic, each level is a ten fold change. most biological fluids are in the range 6-8. if it is greater than seven it is basic, neutral is seven, and less than seven os acidic
acid
any substance that donates protons or hydrogen ions to an aqueous solution, which increases the hydronium ion concentration